Unveiling the crystal structure of thermostable dienelactone hydrolase exhibiting activity on terephthalate esters.
ALMEIDA, Dnane Vieira; CIANCAGLINI, Iara; SANDANO, Ana Luiza Hernandes; ROMAN, Ellen Karen Barreto; ANDRADE, Viviane Brito; NUNES, Ana Bárbara; TRAMONTINA, Robson; SILVA, Viviam Moura da; GABEL, Frank; CORRÊA, Thamy Lívia Ribeiro; DAMÁSIO, André Ricardo de Lima; MUNIZ, João Renato Carvalho; SQUINA, Fabio Marcio; GARCIA, Wânius J.
ALMEIDA, Dnane Vieira; CIANCAGLINI, Iara; SANDANO, Ana Luiza Hernandes; ROMAN, Ellen Karen Barreto; ANDRADE, Viviane Brito; NUNES, Ana Bárbara; TRAMONTINA, Robson; SILVA, Viviam Moura da; GABEL, Frank; CORRÊA, Thamy Lívia Ribeiro; DAMÁSIO, André Ricardo de Lima; MUNIZ, João Renato Carvalho; SQUINA, Fabio Marcio; GARCIA, Wânius J.




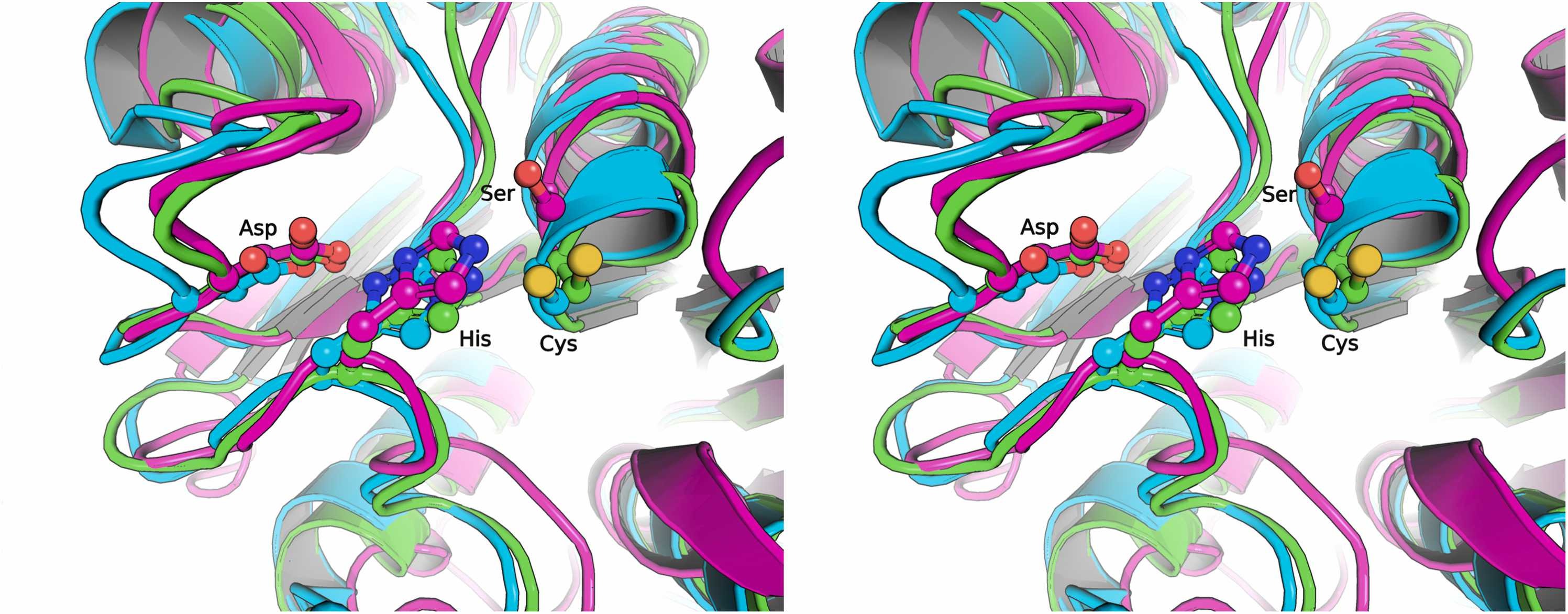 Abstract: Dienelactone hydrolase (DLH) is one of numerous hydrolytic enzymes with an a/ß-hydrolase fold, which catalyze the hydrolysis of dienelactone to maleylacetate. The DLHs share remarkably similar tertiary structures and a conserved arrangement of catalytic residues. This study presents the crystal structure and comprehensive functional characterization of a novel thermostable DLH from the bacterium Hydrogenobacter thermophilus (HtDLH). The crystal structure of the HtDLH, solved at a resolution of about 1.67 Å, exhibits a canonical a/ß-hydrolase fold formed by eight ß-sheet strands in the core, with one buried a-helix and six others exposed to the solvent. The structure also confirmed the conserved catalytic triad of DHLs formed by Cys121, Asp170, and His202 residues. The HtDLH forms stable homodimers in solution. Functional studies showed that HtDLH has the expected esterase activity over esters with short carbon chains, such as p-nitrophenyl acetate, reaching optimal activity at pH 7.5 and 70 °C. Furthermore, HtDLH maintains more than 50 % of its activity even after incubation at 90 °C for 16 h. Interestingly, HtDLH exhibits catalytic activity towards polyethylene terephthalate (PET) monomers, including bis-1,2-hydroxyethyl terephthalate (BHET) and 1-(2-hydroxyethyl) 4-methyl terephthalate, as well as other aliphatic and aromatic esters. These findings associated with the lack of activity on amorphous PET indicate that HtDLH has characteristic of a BHET-degrading enzyme. This work expands our understanding of enzyme families involved in PET degradation, providing novel insights for plastic biorecycling through protein engineering, which could lead to eco-friendly solutions to reduce the accumulation of plastic in landfills and natural environments.
Abstract: Dienelactone hydrolase (DLH) is one of numerous hydrolytic enzymes with an a/ß-hydrolase fold, which catalyze the hydrolysis of dienelactone to maleylacetate. The DLHs share remarkably similar tertiary structures and a conserved arrangement of catalytic residues. This study presents the crystal structure and comprehensive functional characterization of a novel thermostable DLH from the bacterium Hydrogenobacter thermophilus (HtDLH). The crystal structure of the HtDLH, solved at a resolution of about 1.67 Å, exhibits a canonical a/ß-hydrolase fold formed by eight ß-sheet strands in the core, with one buried a-helix and six others exposed to the solvent. The structure also confirmed the conserved catalytic triad of DHLs formed by Cys121, Asp170, and His202 residues. The HtDLH forms stable homodimers in solution. Functional studies showed that HtDLH has the expected esterase activity over esters with short carbon chains, such as p-nitrophenyl acetate, reaching optimal activity at pH 7.5 and 70 °C. Furthermore, HtDLH maintains more than 50 % of its activity even after incubation at 90 °C for 16 h. Interestingly, HtDLH exhibits catalytic activity towards polyethylene terephthalate (PET) monomers, including bis-1,2-hydroxyethyl terephthalate (BHET) and 1-(2-hydroxyethyl) 4-methyl terephthalate, as well as other aliphatic and aromatic esters. These findings associated with the lack of activity on amorphous PET indicate that HtDLH has characteristic of a BHET-degrading enzyme. This work expands our understanding of enzyme families involved in PET degradation, providing novel insights for plastic biorecycling through protein engineering, which could lead to eco-friendly solutions to reduce the accumulation of plastic in landfills and natural environments. @article={003211427,author = {ALMEIDA, Dnane Vieira; CIANCAGLINI, Iara; SANDANO, Ana Luiza Hernandes; ROMAN, Ellen Karen Barreto; ANDRADE, Viviane Brito; NUNES, Ana Bárbara; TRAMONTINA, Robson; SILVA, Viviam Moura da; GABEL, Frank; CORRÊA, Thamy Lívia Ribeiro; DAMÁSIO, André Ricardo de Lima; MUNIZ, João Renato Carvalho; SQUINA, Fabio Marcio; GARCIA, Wânius J.},title={Unveiling the crystal structure of thermostable dienelactone hydrolase exhibiting activity on terephthalate esters},journal={Enzyme and Microbial Technology},note={v. 180, p. 110498-1-110498-10 + supplementary material},year={2024}}
@article={003211427,author = {ALMEIDA, Dnane Vieira; CIANCAGLINI, Iara; SANDANO, Ana Luiza Hernandes; ROMAN, Ellen Karen Barreto; ANDRADE, Viviane Brito; NUNES, Ana Bárbara; TRAMONTINA, Robson; SILVA, Viviam Moura da; GABEL, Frank; CORRÊA, Thamy Lívia Ribeiro; DAMÁSIO, André Ricardo de Lima; MUNIZ, João Renato Carvalho; SQUINA, Fabio Marcio; GARCIA, Wânius J.},title={Unveiling the crystal structure of thermostable dienelactone hydrolase exhibiting activity on terephthalate esters},journal={Enzyme and Microbial Technology},note={v. 180, p. 110498-1-110498-10 + supplementary material},year={2024}}