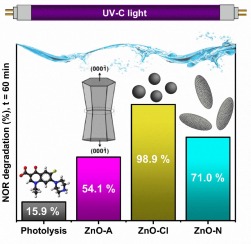
 Abstract: The indiscriminate production and use of antibiotics have caused an environmental imbalance. Therefore, advanced oxidative processes such as photocatalysis have been proposed to eliminate the presence of such emerging pollutants. On the other hand, the use of photocatalytic processes requires the development of highly efficient photocatalysts. This paper introduces photocatalysts based on ZnO nanostructures with different morphologies and grown by low-temperature hydrothermal synthesis. The choice of zinc precursor resulted in significant variations in both the morphology and size of the ZnO particles. Morphologies, such as, dumbbell-like structures, nanogranules, and ellipsoidal particles were obtained by altering the zinc precursor in a slightly alkaline medium, and parameters such as photocatalyst dosage, initial concentration of antibiotic Norfloxacin (NOR), and pH in photocatalysis were thoroughly investigated. The reactive species responsible for NOR degradation were photogenerated holes, superoxide radicals, and hydroxyl radicals, with the latter showing a greater contribution. Degradation pathways are also proposed, and intermediate products were identified by LC-MS. Thirteen degradation products, of which six were reported for the first time, were detected. Among the synthesized morphologies, nanogranules exhibited superior photocatalytic activity, achieving 100% NOR degradation after 70 minutes under UV-C light and excellent reusability after three cycles (97%). The results surpass those from previous studies, since the simple and efficient photocatalysts employed led to shorter degradation times Abstract: The indiscriminate production and use of antibiotics have caused an environmental imbalance. Therefore, advanced oxidative processes such as photocatalysis have been proposed to eliminate the presence of such emerging pollutants. On the other hand, the use of photocatalytic processes requires the development of highly efficient photocatalysts. This paper introduces photocatalysts based on ZnO nanostructures with different morphologies and grown by low-temperature hydrothermal synthesis. The choice of zinc precursor resulted in significant variations in both the morphology and size of the ZnO particles. Morphologies, such as, dumbbell-like structures, nanogranules, and ellipsoidal particles were obtained by altering the zinc precursor in a slightly alkaline medium, and parameters such as photocatalyst dosage, initial concentration of antibiotic Norfloxacin (NOR), and pH in photocatalysis were thoroughly investigated. The reactive species responsible for NOR degradation were photogenerated holes, superoxide radicals, and hydroxyl radicals, with the latter showing a greater contribution. Degradation pathways are also proposed, and intermediate products were identified by LC-MS. Thirteen degradation products, of which six were reported for the first time, were detected. Among the synthesized morphologies, nanogranules exhibited superior photocatalytic activity, achieving 100% NOR degradation after 70 minutes under UV-C light and excellent reusability after three cycles (97%). The results surpass those from previous studies, since the simple and efficient photocatalysts employed led to shorter degradation times |




 Abstract: The indiscriminate production and use of antibiotics have caused an environmental imbalance. Therefore, advanced oxidative processes such as photocatalysis have been proposed to eliminate the presence of such emerging pollutants. On the other hand, the use of photocatalytic processes requires the development of highly efficient photocatalysts. This paper introduces photocatalysts based on ZnO nanostructures with different morphologies and grown by low-temperature hydrothermal synthesis. The choice of zinc precursor resulted in significant variations in both the morphology and size of the ZnO particles. Morphologies, such as, dumbbell-like structures, nanogranules, and ellipsoidal particles were obtained by altering the zinc precursor in a slightly alkaline medium, and parameters such as photocatalyst dosage, initial concentration of antibiotic Norfloxacin (NOR), and pH in photocatalysis were thoroughly investigated. The reactive species responsible for NOR degradation were photogenerated holes, superoxide radicals, and hydroxyl radicals, with the latter showing a greater contribution. Degradation pathways are also proposed, and intermediate products were identified by LC-MS. Thirteen degradation products, of which six were reported for the first time, were detected. Among the synthesized morphologies, nanogranules exhibited superior photocatalytic activity, achieving 100% NOR degradation after 70 minutes under UV-C light and excellent reusability after three cycles (97%). The results surpass those from previous studies, since the simple and efficient photocatalysts employed led to shorter degradation times
Abstract: The indiscriminate production and use of antibiotics have caused an environmental imbalance. Therefore, advanced oxidative processes such as photocatalysis have been proposed to eliminate the presence of such emerging pollutants. On the other hand, the use of photocatalytic processes requires the development of highly efficient photocatalysts. This paper introduces photocatalysts based on ZnO nanostructures with different morphologies and grown by low-temperature hydrothermal synthesis. The choice of zinc precursor resulted in significant variations in both the morphology and size of the ZnO particles. Morphologies, such as, dumbbell-like structures, nanogranules, and ellipsoidal particles were obtained by altering the zinc precursor in a slightly alkaline medium, and parameters such as photocatalyst dosage, initial concentration of antibiotic Norfloxacin (NOR), and pH in photocatalysis were thoroughly investigated. The reactive species responsible for NOR degradation were photogenerated holes, superoxide radicals, and hydroxyl radicals, with the latter showing a greater contribution. Degradation pathways are also proposed, and intermediate products were identified by LC-MS. Thirteen degradation products, of which six were reported for the first time, were detected. Among the synthesized morphologies, nanogranules exhibited superior photocatalytic activity, achieving 100% NOR degradation after 70 minutes under UV-C light and excellent reusability after three cycles (97%). The results surpass those from previous studies, since the simple and efficient photocatalysts employed led to shorter degradation times @article={003206479,author = {LEITE, Ramon Resende; COLOMBO, Renata; BIMBI JUNIOR, Fausto Eduardo; LANZA, Marcos Roberto de Vasconcelos; BARUD, Hernane da Silva; AFONSO, Conrado Ramos Moreira; BERNARDI, Maria Inês Basso.},title={Precursor effect on the hydrothermal synthesis of pure ZnO nanostructures and enhanced photocatalytic performance for norfloxacin degradation},journal={Chemical Engineering Journal},note={v. 496, p. 154374-1-154374-19},year={2024}}
@article={003206479,author = {LEITE, Ramon Resende; COLOMBO, Renata; BIMBI JUNIOR, Fausto Eduardo; LANZA, Marcos Roberto de Vasconcelos; BARUD, Hernane da Silva; AFONSO, Conrado Ramos Moreira; BERNARDI, Maria Inês Basso.},title={Precursor effect on the hydrothermal synthesis of pure ZnO nanostructures and enhanced photocatalytic performance for norfloxacin degradation},journal={Chemical Engineering Journal},note={v. 496, p. 154374-1-154374-19},year={2024}}