Unveiling a strategy for ring opening of epoxides: synthesis of 2-hydroxyindolinylidenes using a-ester sulfoxonium ylides.
CÂMARA, Viktor Saraiva; SILVA, Aislan Leme da; LUZ, Lilian Camargo da; RODEMBUSCH, Fabiano Severo; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; BURTOLOSO, Antonio Carlos Bender.
CÂMARA, Viktor Saraiva; SILVA, Aislan Leme da; LUZ, Lilian Camargo da; RODEMBUSCH, Fabiano Severo; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; BURTOLOSO, Antonio Carlos Bender.




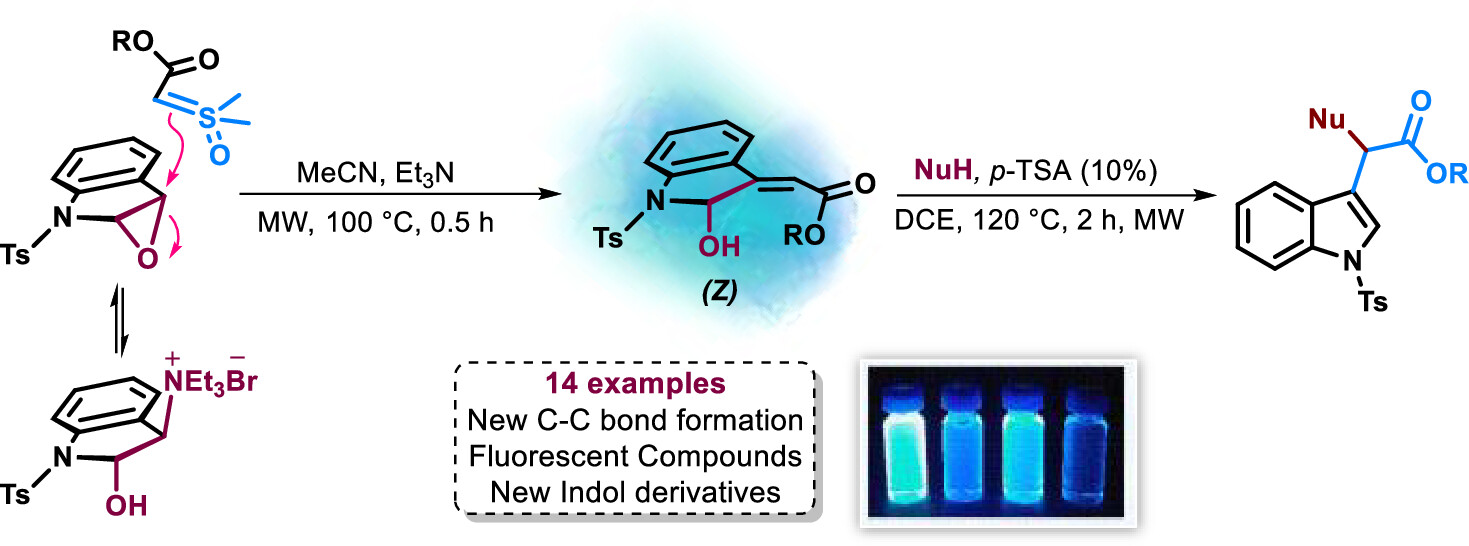 Abstract: The untapped potential of a-carbonyl sulfoxonium ylides in epoxide ring-opening reactions has been a notable gap in current research, with such reactivity predominantly associated with the highly reactive dimethylsulfoxonium methylide. This study introduces an innovative approach wherein an epoxide indole, formed in situ from 2-hydroxyindoline-3-triethylammonium bromide, undergoes reaction with a-ester sulfoxonium ylides. The outcome is the efficient synthesis of a range of 2-hydroxyindolin-3-ylidenes, demonstrating favorable yields (41-81%) and Z/E ratios from 4:1 to those of exclusive Z isomers. Additionally, the photophysical properties of the synthesized indolinylidenes are explored, along with their derivatization using various nucleophiles under acid catalysis.
Abstract: The untapped potential of a-carbonyl sulfoxonium ylides in epoxide ring-opening reactions has been a notable gap in current research, with such reactivity predominantly associated with the highly reactive dimethylsulfoxonium methylide. This study introduces an innovative approach wherein an epoxide indole, formed in situ from 2-hydroxyindoline-3-triethylammonium bromide, undergoes reaction with a-ester sulfoxonium ylides. The outcome is the efficient synthesis of a range of 2-hydroxyindolin-3-ylidenes, demonstrating favorable yields (41-81%) and Z/E ratios from 4:1 to those of exclusive Z isomers. Additionally, the photophysical properties of the synthesized indolinylidenes are explored, along with their derivatization using various nucleophiles under acid catalysis. @article={003182205,author = {CÂMARA, Viktor Saraiva; SILVA, Aislan Leme da; LUZ, Lilian Camargo da; RODEMBUSCH, Fabiano Severo; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; BURTOLOSO, Antonio Carlos Bender.},title={Unveiling a strategy for ring opening of epoxides: synthesis of 2-hydroxyindolinylidenes using a-ester sulfoxonium ylides},journal={Organic Letters},note={v. 26, n. 5, p. 1034-1039 + supporting information: 1-61},year={2024}}
@article={003182205,author = {CÂMARA, Viktor Saraiva; SILVA, Aislan Leme da; LUZ, Lilian Camargo da; RODEMBUSCH, Fabiano Severo; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; BURTOLOSO, Antonio Carlos Bender.},title={Unveiling a strategy for ring opening of epoxides: synthesis of 2-hydroxyindolinylidenes using a-ester sulfoxonium ylides},journal={Organic Letters},note={v. 26, n. 5, p. 1034-1039 + supporting information: 1-61},year={2024}}