An in-solution snapshot of SARS-COV-2main protease maturation process and inhibition.
NOSKE, Gabriela Dias; SONG, Yun; FERNANDES, Rafaela Sachetto; CHALK, Rod; ELMASSOUDI, Haitem; KOEKEMOER, Lizbé; OWEN, Christopher David; EL-BABA, Tarick J.; ROBINSON, Dame Carol; OLIVA, Glaucius; GODOY, Andre Schutzer.
NOSKE, Gabriela Dias; SONG, Yun; FERNANDES, Rafaela Sachetto; CHALK, Rod; ELMASSOUDI, Haitem; KOEKEMOER, Lizbé; OWEN, Christopher David; EL-BABA, Tarick J.; ROBINSON, Dame Carol; OLIVA, Glaucius; GODOY, Andre Schutzer.




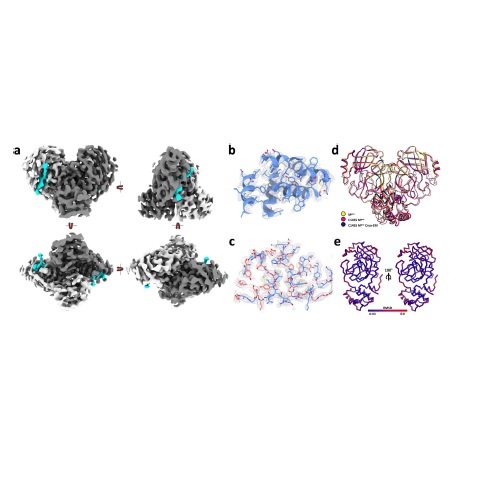 Abstract: The main protease from SARS-CoV-2 (Mpro) is responsible for cleavage of the viral polyprotein. Mpro self-processing is called maturation, and it is crucial for enzyme dimerization and activity. Here we use C145S Mpro to study the structure and dynamics of N-terminal cleavage in solution. Native mass spectroscopy analysis shows that mixed oligomeric states are composed of cleaved and uncleaved particles, indicating that N-terminal processing is not critical for dimerization. A 3.5 Å cryo-EM structure provides details of Mpro N-terminal cleavage outside the constrains of crystal environment. We showthat different classes of inhibitors shift the balance between oligomeric states. While noncovalent inhibitor MAT-POS-e194df51-1 prevents dimerization, the covalent inhibitor nirmatrelvir induces the conversion of monomers into dimers, even with intact N-termini. Our data indicates that theMpro dimerization is triggered by induced fit due to covalent linkage during substrate processing rather than the N-terminal processing.
Abstract: The main protease from SARS-CoV-2 (Mpro) is responsible for cleavage of the viral polyprotein. Mpro self-processing is called maturation, and it is crucial for enzyme dimerization and activity. Here we use C145S Mpro to study the structure and dynamics of N-terminal cleavage in solution. Native mass spectroscopy analysis shows that mixed oligomeric states are composed of cleaved and uncleaved particles, indicating that N-terminal processing is not critical for dimerization. A 3.5 Å cryo-EM structure provides details of Mpro N-terminal cleavage outside the constrains of crystal environment. We showthat different classes of inhibitors shift the balance between oligomeric states. While noncovalent inhibitor MAT-POS-e194df51-1 prevents dimerization, the covalent inhibitor nirmatrelvir induces the conversion of monomers into dimers, even with intact N-termini. Our data indicates that theMpro dimerization is triggered by induced fit due to covalent linkage during substrate processing rather than the N-terminal processing. @article={003127907,author = {NOSKE, Gabriela Dias; SONG, Yun; FERNANDES, Rafaela Sachetto; CHALK, Rod; ELMASSOUDI, Haitem; KOEKEMOER, Lizbé; OWEN, Christopher David; EL-BABA, Tarick J.; ROBINSON, Dame Carol; OLIVA, Glaucius; GODOY, Andre Schutzer.},title={An in-solution snapshot of SARS-COV-2main protease maturation process and inhibition},journal={Nature Communications},note={v. 14, p. 1545-1-1545-13 + supplementary information},year={2022}}
@article={003127907,author = {NOSKE, Gabriela Dias; SONG, Yun; FERNANDES, Rafaela Sachetto; CHALK, Rod; ELMASSOUDI, Haitem; KOEKEMOER, Lizbé; OWEN, Christopher David; EL-BABA, Tarick J.; ROBINSON, Dame Carol; OLIVA, Glaucius; GODOY, Andre Schutzer.},title={An in-solution snapshot of SARS-COV-2main protease maturation process and inhibition},journal={Nature Communications},note={v. 14, p. 1545-1-1545-13 + supplementary information},year={2022}}