3-arylthioimidazo[1,2-a]pyridine derivatives: a theoretical and experimental study of its photophysical properties.
COCCA, Leandro Henrique Zucolotto; PELOSI, André Gasparotto; VALVERDE, João Victor Pereira; BESCONT, Julie le; BRETON-PATIENT, Chloé; PIGUEL, Sandrine; MENDONÇA, Cleber Renato; DE BONI, Leonardo.
COCCA, Leandro Henrique Zucolotto; PELOSI, André Gasparotto; VALVERDE, João Victor Pereira; BESCONT, Julie le; BRETON-PATIENT, Chloé; PIGUEL, Sandrine; MENDONÇA, Cleber Renato; DE BONI, Leonardo.




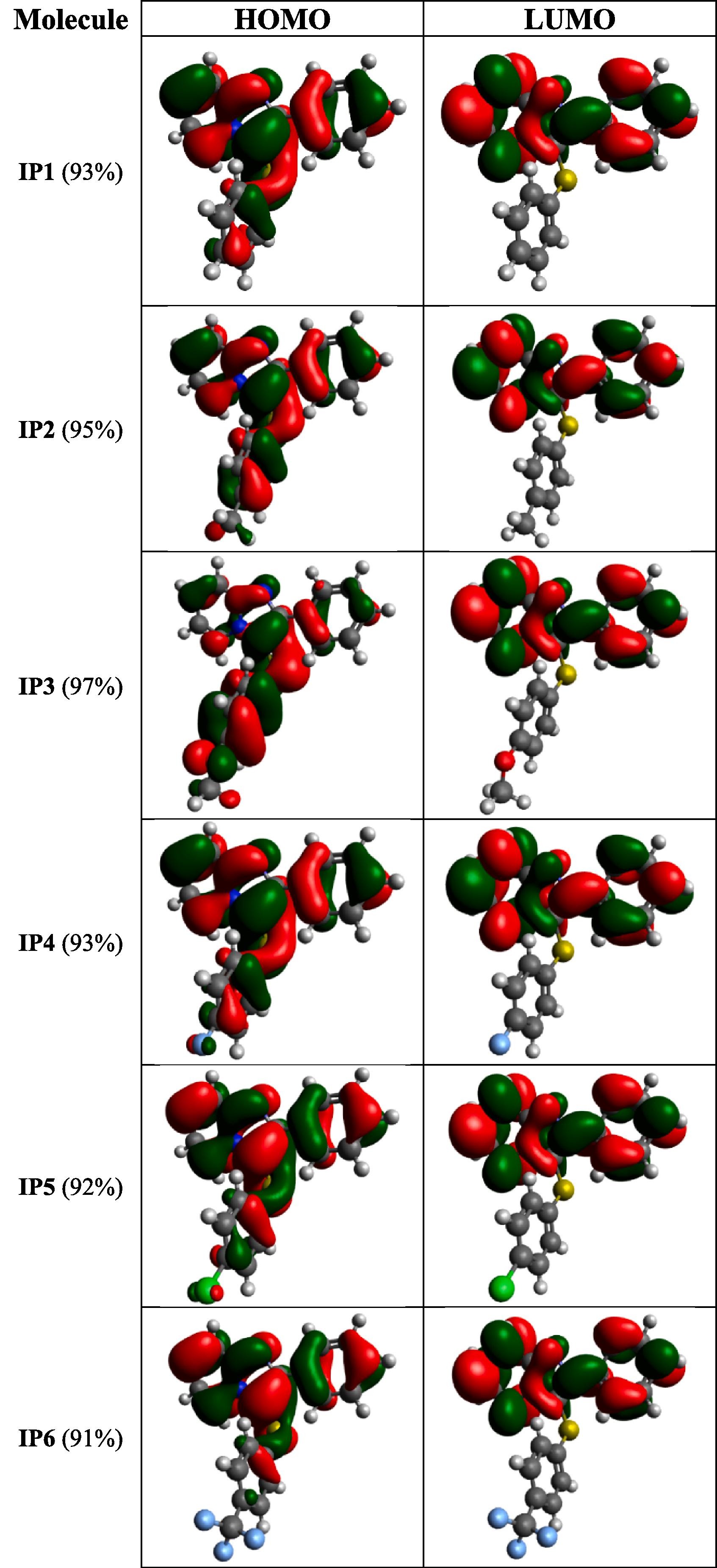 Abstract: The optical properties of imidazo[1,2-a]pyridines possessing various arylthiol substituents at position 3 are described for the first time. Several optical spectroscopy studies were performed to investigate the influence of the electronic effects of the substituents on the thioaryl group and the influence of the sulfur atom as a linker. The compounds exhibited two absorption bands, which are related to the p?p* transitions, and wide Stokes shift of ca. 100 nm were obtained owing to a substantial electronic modification in the excited state. The fluorescence quantum yield increased by 90% when replacing the trifluoromethyl peripheral group with fluorine. Moreover, it was observed that permanent dipole moment values increase as strength electron-donating or electronwithdrawing substituents groups, which is associate to Hammett constant. In conclusion, this experimental and theoretical study revealed a possible way in view of to optimize the 3-arylthioimidazo[1,2-a]pyridine derivatives optical properties through peripherals groups substitution.
Abstract: The optical properties of imidazo[1,2-a]pyridines possessing various arylthiol substituents at position 3 are described for the first time. Several optical spectroscopy studies were performed to investigate the influence of the electronic effects of the substituents on the thioaryl group and the influence of the sulfur atom as a linker. The compounds exhibited two absorption bands, which are related to the p?p* transitions, and wide Stokes shift of ca. 100 nm were obtained owing to a substantial electronic modification in the excited state. The fluorescence quantum yield increased by 90% when replacing the trifluoromethyl peripheral group with fluorine. Moreover, it was observed that permanent dipole moment values increase as strength electron-donating or electronwithdrawing substituents groups, which is associate to Hammett constant. In conclusion, this experimental and theoretical study revealed a possible way in view of to optimize the 3-arylthioimidazo[1,2-a]pyridine derivatives optical properties through peripherals groups substitution. @article={003126205,author = {COCCA, Leandro Henrique Zucolotto; PELOSI, André Gasparotto; VALVERDE, João Victor Pereira; BESCONT, Julie le; BRETON-PATIENT, Chloé; PIGUEL, Sandrine; MENDONÇA, Cleber Renato; DE BONI, Leonardo.},title={3-arylthioimidazo[1,2-a]pyridine derivatives: a theoretical and experimental study of its photophysical properties},journal={Journal of Photochemistry and Photobiology A},note={v. 440, p. 114675-1-114675-10 + supplementary data},year={2023}}
@article={003126205,author = {COCCA, Leandro Henrique Zucolotto; PELOSI, André Gasparotto; VALVERDE, João Victor Pereira; BESCONT, Julie le; BRETON-PATIENT, Chloé; PIGUEL, Sandrine; MENDONÇA, Cleber Renato; DE BONI, Leonardo.},title={3-arylthioimidazo[1,2-a]pyridine derivatives: a theoretical and experimental study of its photophysical properties},journal={Journal of Photochemistry and Photobiology A},note={v. 440, p. 114675-1-114675-10 + supplementary data},year={2023}}