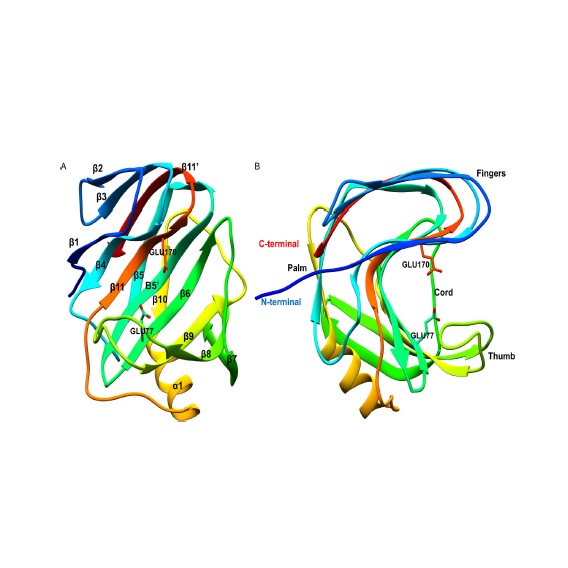
 Abstract: Glycoside hydrolases (GHs) are essential for plant biomass deconstruction. GH11 family consist of endob- 1,4-xylanases which hydrolyze xylan, the second most abundant cell wall biopolymer after cellulose, into small bioavailable oligomers. Structural requirements for enzymatic mechanism of xylan hydrolysis is well described for GH11 members. However, over the last years, it has been discovered that some enzymes from GH11 family have a secondary binding sites (SBS), which modulate the enzymes activities, but mechanistic details of the molecular communication between the active site and SBS of the enzymes remain a conundrum. In the present work we structurally characterized GH11 xylanase from Paenibacillus xylanivorans A57 (PxXyn11B), a microorganism of agricultural importance, using protein crystallography and molecular dynamics simulations. The PxXyn11B structure was solved to 2.5 Å resolution and different substrates (xylo-oligosaccharides from X3 to X6), were modelled in its active and SBS sites. Molecular Dynamics (MD) simulations revealed an important role of SBS in the activity and conformational mobility of PxXyn11B, demonstrating that binding of the reaction products to the SBS of the enzyme stabilizes the N-terminal region and, consequently, the active site. Furthermore, MD simulations showed that the longer the ligand, the better is the stabilization within active site, and the positive subsites contribute less to the stabilization of the substrates than the negative ones. These findings provide rationale for the observed enzyme kinetics, shedding light on the conformational modulation of the GH11 enzymes via their SBS mediated by the positive molecular feedback loop which involve the products of the enzymatic reaction. Abstract: Glycoside hydrolases (GHs) are essential for plant biomass deconstruction. GH11 family consist of endob- 1,4-xylanases which hydrolyze xylan, the second most abundant cell wall biopolymer after cellulose, into small bioavailable oligomers. Structural requirements for enzymatic mechanism of xylan hydrolysis is well described for GH11 members. However, over the last years, it has been discovered that some enzymes from GH11 family have a secondary binding sites (SBS), which modulate the enzymes activities, but mechanistic details of the molecular communication between the active site and SBS of the enzymes remain a conundrum. In the present work we structurally characterized GH11 xylanase from Paenibacillus xylanivorans A57 (PxXyn11B), a microorganism of agricultural importance, using protein crystallography and molecular dynamics simulations. The PxXyn11B structure was solved to 2.5 Å resolution and different substrates (xylo-oligosaccharides from X3 to X6), were modelled in its active and SBS sites. Molecular Dynamics (MD) simulations revealed an important role of SBS in the activity and conformational mobility of PxXyn11B, demonstrating that binding of the reaction products to the SBS of the enzyme stabilizes the N-terminal region and, consequently, the active site. Furthermore, MD simulations showed that the longer the ligand, the better is the stabilization within active site, and the positive subsites contribute less to the stabilization of the substrates than the negative ones. These findings provide rationale for the observed enzyme kinetics, shedding light on the conformational modulation of the GH11 enzymes via their SBS mediated by the positive molecular feedback loop which involve the products of the enzymatic reaction. |




 Abstract: Glycoside hydrolases (GHs) are essential for plant biomass deconstruction. GH11 family consist of endob- 1,4-xylanases which hydrolyze xylan, the second most abundant cell wall biopolymer after cellulose, into small bioavailable oligomers. Structural requirements for enzymatic mechanism of xylan hydrolysis is well described for GH11 members. However, over the last years, it has been discovered that some enzymes from GH11 family have a secondary binding sites (SBS), which modulate the enzymes activities, but mechanistic details of the molecular communication between the active site and SBS of the enzymes remain a conundrum. In the present work we structurally characterized GH11 xylanase from Paenibacillus xylanivorans A57 (PxXyn11B), a microorganism of agricultural importance, using protein crystallography and molecular dynamics simulations. The PxXyn11B structure was solved to 2.5 Å resolution and different substrates (xylo-oligosaccharides from X3 to X6), were modelled in its active and SBS sites. Molecular Dynamics (MD) simulations revealed an important role of SBS in the activity and conformational mobility of PxXyn11B, demonstrating that binding of the reaction products to the SBS of the enzyme stabilizes the N-terminal region and, consequently, the active site. Furthermore, MD simulations showed that the longer the ligand, the better is the stabilization within active site, and the positive subsites contribute less to the stabilization of the substrates than the negative ones. These findings provide rationale for the observed enzyme kinetics, shedding light on the conformational modulation of the GH11 enzymes via their SBS mediated by the positive molecular feedback loop which involve the products of the enzymatic reaction.
Abstract: Glycoside hydrolases (GHs) are essential for plant biomass deconstruction. GH11 family consist of endob- 1,4-xylanases which hydrolyze xylan, the second most abundant cell wall biopolymer after cellulose, into small bioavailable oligomers. Structural requirements for enzymatic mechanism of xylan hydrolysis is well described for GH11 members. However, over the last years, it has been discovered that some enzymes from GH11 family have a secondary binding sites (SBS), which modulate the enzymes activities, but mechanistic details of the molecular communication between the active site and SBS of the enzymes remain a conundrum. In the present work we structurally characterized GH11 xylanase from Paenibacillus xylanivorans A57 (PxXyn11B), a microorganism of agricultural importance, using protein crystallography and molecular dynamics simulations. The PxXyn11B structure was solved to 2.5 Å resolution and different substrates (xylo-oligosaccharides from X3 to X6), were modelled in its active and SBS sites. Molecular Dynamics (MD) simulations revealed an important role of SBS in the activity and conformational mobility of PxXyn11B, demonstrating that binding of the reaction products to the SBS of the enzyme stabilizes the N-terminal region and, consequently, the active site. Furthermore, MD simulations showed that the longer the ligand, the better is the stabilization within active site, and the positive subsites contribute less to the stabilization of the substrates than the negative ones. These findings provide rationale for the observed enzyme kinetics, shedding light on the conformational modulation of the GH11 enzymes via their SBS mediated by the positive molecular feedback loop which involve the products of the enzymatic reaction. @article={003024499,author = {BRIGANTI, Lorenzo; CAPETTI, Caio Cesar de Mello; PELLEGRINI, Vanessa de Oliveira Arnoldi; GHIO, Silvina; CAMPOS, Eleonora; NASCIMENTO, Alessandro Silva; POLIKARPOV, Igor.},title={Structural and molecular dynamics investigations of ligand stabilization via secondary binding site interactions in Paenibacillus xylanivorans GH11 xylanase},journal={Computational and Structural Biotechnology Journal},note={v. 19, p. 1557-1566},year={2021}}
@article={003024499,author = {BRIGANTI, Lorenzo; CAPETTI, Caio Cesar de Mello; PELLEGRINI, Vanessa de Oliveira Arnoldi; GHIO, Silvina; CAMPOS, Eleonora; NASCIMENTO, Alessandro Silva; POLIKARPOV, Igor.},title={Structural and molecular dynamics investigations of ligand stabilization via secondary binding site interactions in Paenibacillus xylanivorans GH11 xylanase},journal={Computational and Structural Biotechnology Journal},note={v. 19, p. 1557-1566},year={2021}}