Chalcones and their B-aryl analogues as myeloperoxidase inhibitors: in silico, in vitro and ex vivo investigations.
SANTOS, Mariana Bastos dos; MARQUES, Beatriz Carvalho; AYUSSO, Gabriela Miranda; GARCIA, Mayara Aparecida Rocha; PARACATU, Luana Chiquetto; PAULI, Ivani; BOLZANI, Vanderlan da Silva; ANDRICOPULO, Adriano Defini; XIMENES, Valdecir Farias; ZERAIK, Maria Luiza; REGASINI, Luis Octavio.
SANTOS, Mariana Bastos dos; MARQUES, Beatriz Carvalho; AYUSSO, Gabriela Miranda; GARCIA, Mayara Aparecida Rocha; PARACATU, Luana Chiquetto; PAULI, Ivani; BOLZANI, Vanderlan da Silva; ANDRICOPULO, Adriano Defini; XIMENES, Valdecir Farias; ZERAIK, Maria Luiza; REGASINI, Luis Octavio.




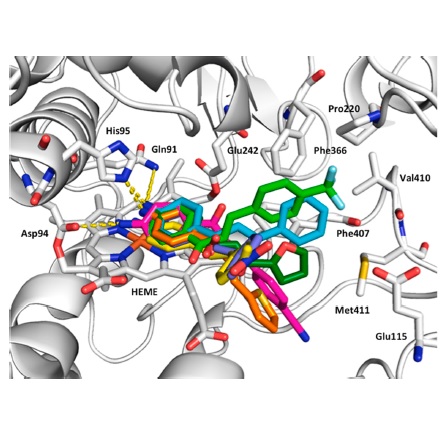 Abstract: In the present study, a series of chalcones and their B-aryl analogues were prepared and evaluate as inhibitors of myeloperoxidase (MPO) chlorinating activity, using in vitro and ex vivo assays. Among these, B-thiophenyl chalcone (analogue 9) demonstrated inhibition of in vitro and ex vivo MPO chlorinating activity, exhibiting IC50 value of 0.53 and 19.2 µM, respectively. Potent ex vivo MPO inhibitors 5, 8 and 9 were not toxic to human neutrophils at 50 µM, as well as displayed weak 2,2-diphenyl-1-pycrylhydrazyl radical (DPPH?) and hypochlorous acid (HOCl) scavenger abilities. Docking simulations indicated binding mode of MPO inhibitors, evidencing hydrogen bonds between the amino group at 4'position (ring A) of chalcones with Gln91, Asp94, and Hys95 MPO residues. In this regard, the efficacy and low toxicity promoted aminochalcones and arylic analogues to the rank of hit compounds in the search for new non-steroidal anti-inflammatory compounds.
Abstract: In the present study, a series of chalcones and their B-aryl analogues were prepared and evaluate as inhibitors of myeloperoxidase (MPO) chlorinating activity, using in vitro and ex vivo assays. Among these, B-thiophenyl chalcone (analogue 9) demonstrated inhibition of in vitro and ex vivo MPO chlorinating activity, exhibiting IC50 value of 0.53 and 19.2 µM, respectively. Potent ex vivo MPO inhibitors 5, 8 and 9 were not toxic to human neutrophils at 50 µM, as well as displayed weak 2,2-diphenyl-1-pycrylhydrazyl radical (DPPH?) and hypochlorous acid (HOCl) scavenger abilities. Docking simulations indicated binding mode of MPO inhibitors, evidencing hydrogen bonds between the amino group at 4'position (ring A) of chalcones with Gln91, Asp94, and Hys95 MPO residues. In this regard, the efficacy and low toxicity promoted aminochalcones and arylic analogues to the rank of hit compounds in the search for new non-steroidal anti-inflammatory compounds. @article={003024203,author = {SANTOS, Mariana Bastos dos; MARQUES, Beatriz Carvalho; AYUSSO, Gabriela Miranda; GARCIA, Mayara Aparecida Rocha; PARACATU, Luana Chiquetto; PAULI, Ivani; BOLZANI, Vanderlan da Silva; ANDRICOPULO, Adriano Defini; XIMENES, Valdecir Farias; ZERAIK, Maria Luiza; REGASINI, Luis Octavio.},title={Chalcones and their B-aryl analogues as myeloperoxidase inhibitors: in silico, in vitro and ex vivo investigations},journal={Bioorganic Chemistry},note={v. 110, p. 104773-1-104773-7},year={2021}}
@article={003024203,author = {SANTOS, Mariana Bastos dos; MARQUES, Beatriz Carvalho; AYUSSO, Gabriela Miranda; GARCIA, Mayara Aparecida Rocha; PARACATU, Luana Chiquetto; PAULI, Ivani; BOLZANI, Vanderlan da Silva; ANDRICOPULO, Adriano Defini; XIMENES, Valdecir Farias; ZERAIK, Maria Luiza; REGASINI, Luis Octavio.},title={Chalcones and their B-aryl analogues as myeloperoxidase inhibitors: in silico, in vitro and ex vivo investigations},journal={Bioorganic Chemistry},note={v. 110, p. 104773-1-104773-7},year={2021}}