Light-stimulated T. thermophilus two-domain LPMO9H: low-resolution SAXS model and synergy with cellulases.
HIGASI, Paula Miwa Rabêlo; VELASCO, Josman; PELLEGRINI, Vanessa de Oliveira Arnoldi; ARAÚJO, Evandro Ares de; FRANÇA, Bruno Alves; KELLER, Malene B; LABATE, Carlos Alberto; BLOSSOM, Benedikt M; SEGATO, Fernando; POLIKARPOV, Igor.
HIGASI, Paula Miwa Rabêlo; VELASCO, Josman; PELLEGRINI, Vanessa de Oliveira Arnoldi; ARAÚJO, Evandro Ares de; FRANÇA, Bruno Alves; KELLER, Malene B; LABATE, Carlos Alberto; BLOSSOM, Benedikt M; SEGATO, Fernando; POLIKARPOV, Igor.




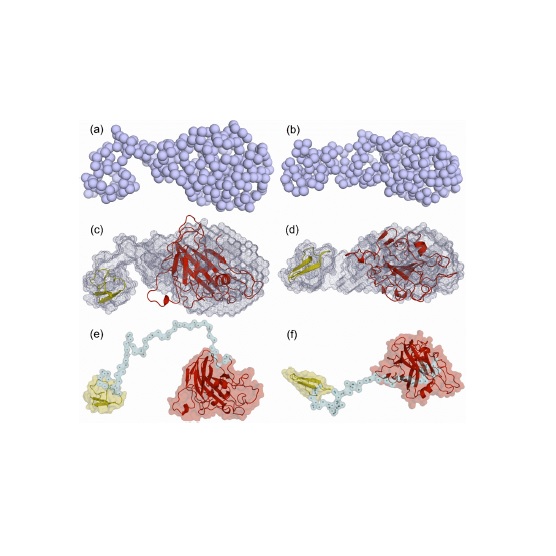 Abstract: Lytic polysaccharide monooxygenases (LPMOs), monocopper enzymes that oxidatively cleave recalcitrant polysaccharides, have important biotechnological applications. Thermothelomyces thermophilus is a rich source of biomass-active enzymes, including many members from auxiliary activities family 9 LPMOs. Here, we report biochemical and structural characterization of recombinant TtLPMO9H which oxidizes cellulose at the C1 and C4 positions and shows enhanced activity in light-driven catalysis assays. TtLPMO9H also shows activity against xyloglucan. The addition of TtLPMO9H to endoglucanases from four different glucoside hydrolase families (GH5, GH12, GH45 and GH7) revealed that the product formation was remarkably increased when TtLPMO9H was combined with GH7 endoglucanase. Finally, we determind the first low resolution small-angle X-ray scattering model of the two-domain TtLPMO9H in solution that shows relative positions of its two functional domains and a conformation of the linker peptide, which can be relevant for the catalytic oxidation of cellulose and xyloglucan.
Abstract: Lytic polysaccharide monooxygenases (LPMOs), monocopper enzymes that oxidatively cleave recalcitrant polysaccharides, have important biotechnological applications. Thermothelomyces thermophilus is a rich source of biomass-active enzymes, including many members from auxiliary activities family 9 LPMOs. Here, we report biochemical and structural characterization of recombinant TtLPMO9H which oxidizes cellulose at the C1 and C4 positions and shows enhanced activity in light-driven catalysis assays. TtLPMO9H also shows activity against xyloglucan. The addition of TtLPMO9H to endoglucanases from four different glucoside hydrolase families (GH5, GH12, GH45 and GH7) revealed that the product formation was remarkably increased when TtLPMO9H was combined with GH7 endoglucanase. Finally, we determind the first low resolution small-angle X-ray scattering model of the two-domain TtLPMO9H in solution that shows relative positions of its two functional domains and a conformation of the linker peptide, which can be relevant for the catalytic oxidation of cellulose and xyloglucan. @article={003021431,author = {HIGASI, Paula Miwa Rabêlo; VELASCO, Josman; PELLEGRINI, Vanessa de Oliveira Arnoldi; ARAÚJO, Evandro Ares de; FRANÇA, Bruno Alves; KELLER, Malene B; LABATE, Carlos Alberto; BLOSSOM, Benedikt M; SEGATO, Fernando; POLIKARPOV, Igor.},title={Light-stimulated T. thermophilus two-domain LPMO9H: low-resolution SAXS model and synergy with cellulases},journal={Carbohydrate Polymers},note={v. 260, art. 117814, p. 1-11},year={2021}}
@article={003021431,author = {HIGASI, Paula Miwa Rabêlo; VELASCO, Josman; PELLEGRINI, Vanessa de Oliveira Arnoldi; ARAÚJO, Evandro Ares de; FRANÇA, Bruno Alves; KELLER, Malene B; LABATE, Carlos Alberto; BLOSSOM, Benedikt M; SEGATO, Fernando; POLIKARPOV, Igor.},title={Light-stimulated T. thermophilus two-domain LPMO9H: low-resolution SAXS model and synergy with cellulases},journal={Carbohydrate Polymers},note={v. 260, art. 117814, p. 1-11},year={2021}}