A novel thermostable GH5 ß-xylosidase from Thermogemmatispora sp. T81.
TOMAZINI JUNIOR, Atilio; HIGASI, Paula Miwa Rabêlo; MANZINE, Livia Regina; STOTT, Matthew; SPARLING, Richard; LEVIN, David B.; POLIKARPOV, Igor.
TOMAZINI JUNIOR, Atilio; HIGASI, Paula Miwa Rabêlo; MANZINE, Livia Regina; STOTT, Matthew; SPARLING, Richard; LEVIN, David B.; POLIKARPOV, Igor.
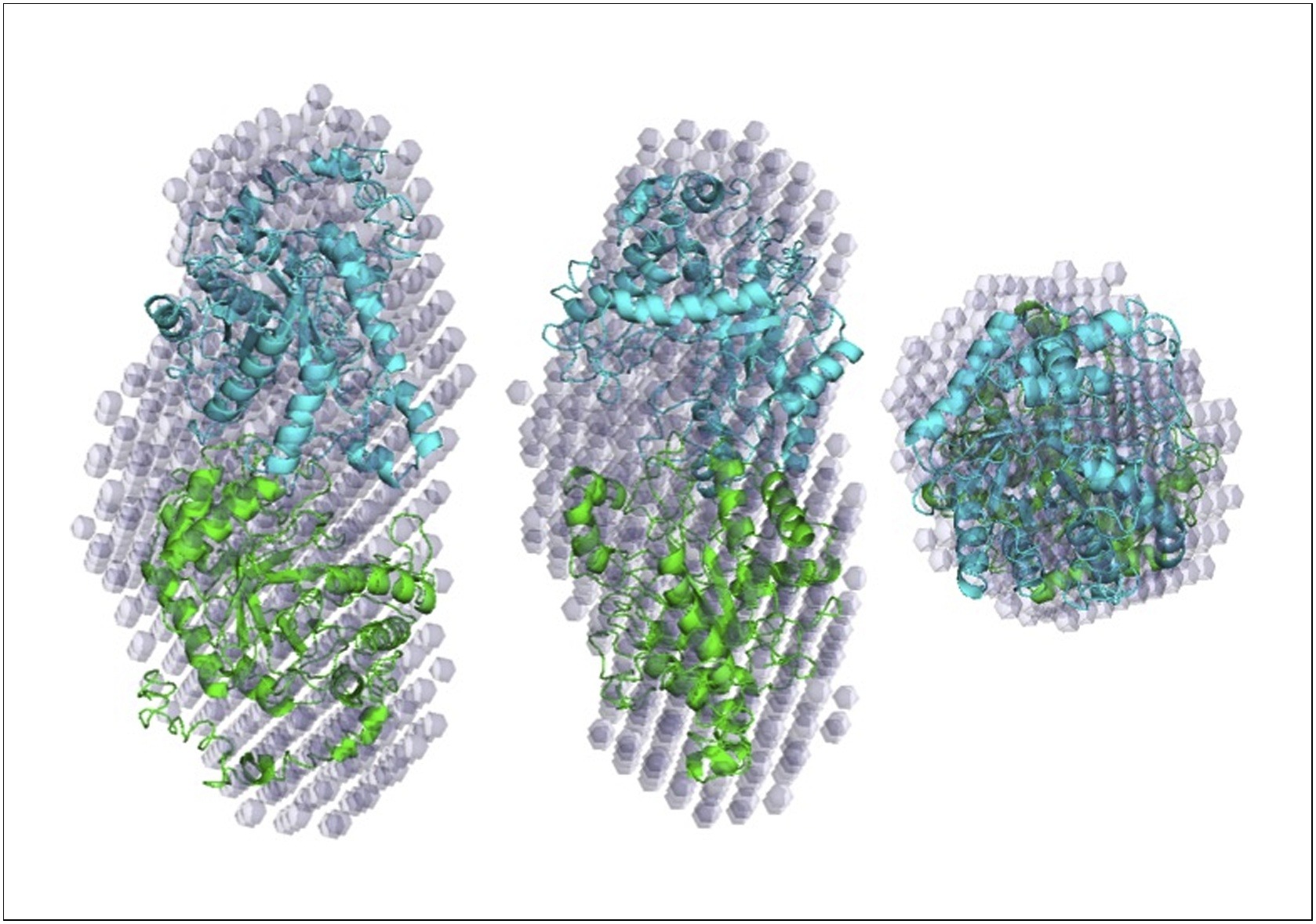 Abstract: A glycoside hydrolase family 5 (GH5) subfamily 22 gene, designated T81Xyl5_22A, was identified in the genome of the aerobic thermophilic bacterium, Thermogemmatispora sp. T81 (locus A4R35_07040). The gene was cloned and heterologously expressed in Escherichia coli and the gene product characterized biochemically. The recombinant enzyme had an optimal catalytic activity at pH5.0 and 65 °C, and was active against beechwood xylan and rye arabinoxylan. It yielded only xylose molecules as products of beechwood xylan hydrolysis, indicating that it is a GH5 family ß-d-xylosidase. Using 4-nitrophenyl ß-d-xylopyranoside (pNPX) as a substrate, the KM, Vmax, kcat and kcat/KM kinetic parameters were determined as 0.25 ± 0.03 mM, 889.47 ± 28.54 U/mg, 39.20 s-1 and 156.8 mM-1s-1, respectively. Small-angle X-ray scattering (SAXS) data enabled reconstruction of the enzyme?s low-resolution molecular envelope and revealed that it formed dimers in solution. As far as we are aware, this is the first description of a thermostable bacterial GH5 family ß-d-xylosidase. Abstract: A glycoside hydrolase family 5 (GH5) subfamily 22 gene, designated T81Xyl5_22A, was identified in the genome of the aerobic thermophilic bacterium, Thermogemmatispora sp. T81 (locus A4R35_07040). The gene was cloned and heterologously expressed in Escherichia coli and the gene product characterized biochemically. The recombinant enzyme had an optimal catalytic activity at pH5.0 and 65 °C, and was active against beechwood xylan and rye arabinoxylan. It yielded only xylose molecules as products of beechwood xylan hydrolysis, indicating that it is a GH5 family ß-d-xylosidase. Using 4-nitrophenyl ß-d-xylopyranoside (pNPX) as a substrate, the KM, Vmax, kcat and kcat/KM kinetic parameters were determined as 0.25 ± 0.03 mM, 889.47 ± 28.54 U/mg, 39.20 s-1 and 156.8 mM-1s-1, respectively. Small-angle X-ray scattering (SAXS) data enabled reconstruction of the enzyme?s low-resolution molecular envelope and revealed that it formed dimers in solution. As far as we are aware, this is the first description of a thermostable bacterial GH5 family ß-d-xylosidase. | |
| New Biotechnology |
| v. 53, p. 57-64 - Ano: 2019 |
| Fator de Impacto: 3,739 |
| http://dx.doi.org/10.1016/j.nbt.2019.07.002 |  @article={002952748,author = {TOMAZINI JUNIOR, Atilio; HIGASI, Paula Miwa Rabêlo; MANZINE, Livia Regina; STOTT, Matthew; SPARLING, Richard; LEVIN, David B.; POLIKARPOV, Igor.},title={A novel thermostable GH5 ß-xylosidase from Thermogemmatispora sp. T81},journal={New Biotechnology},note={v. 53, p. 57-64},year={2019}} @article={002952748,author = {TOMAZINI JUNIOR, Atilio; HIGASI, Paula Miwa Rabêlo; MANZINE, Livia Regina; STOTT, Matthew; SPARLING, Richard; LEVIN, David B.; POLIKARPOV, Igor.},title={A novel thermostable GH5 ß-xylosidase from Thermogemmatispora sp. T81},journal={New Biotechnology},note={v. 53, p. 57-64},year={2019}} |



