Unraveling common patterns and differences among cruzipains through molecular dynamics simulations and structural analyses.
SANTOS, Lucianna Helene Silva; CAMPOS, Augusto César Broilo; SANTOS, Viviane Corrêa; FASSIO, Alexandre Victor; COSTA, Mauricio Garcia de Souza; FERREIRA, Rafaela Salgado.
SANTOS, Lucianna Helene Silva; CAMPOS, Augusto César Broilo; SANTOS, Viviane Corrêa; FASSIO, Alexandre Victor; COSTA, Mauricio Garcia de Souza; FERREIRA, Rafaela Salgado.




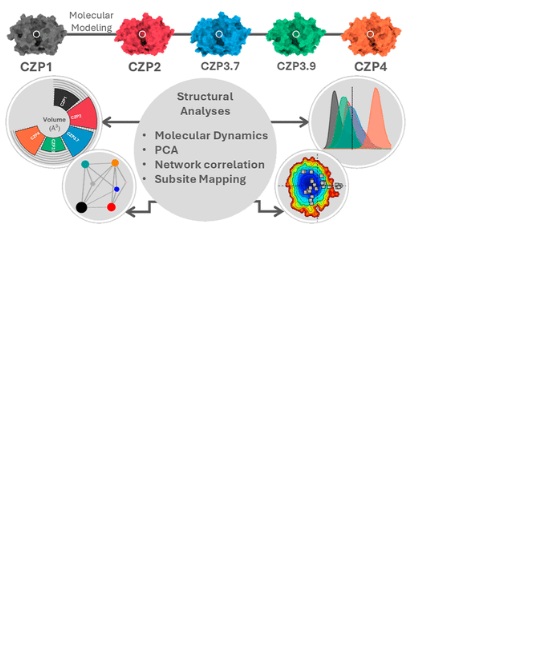 Abstract: Chagas disease (CD) is a neglected tropical disease for which novel and improved treatments are needed. The cysteine protease Cruzipain is one of the main targets for the development of novel drugs for the treatment of CD. Recent bioinformatics analyses have revealed four Cruzipain subtypes whose active sites differ in key positions for ligand recognition. These analyses suggest a possible effect on the substrate specificity and affinity for ligands. To better investigate the impact of substitutions in Cruzipain subtypes, we employed molecular dynamics simulations and varied structural analyses on representatives of each Cruzipain subtype. Our results indicated that the substitutions did not significantly affect the overall flexibility and conformation of these proteases. In contrast, we observed differences in their active site characteristics, including different electrostatic potentials, cavity volumes, and patterns of interactions with the virtual probes. The distinct alterations in the active site subsites, especially in the S2 subsite, suggest unique functional changes that could affect substrate binding, ligand recognition, and possibly enzymatic effectiveness in various biological situations.
Abstract: Chagas disease (CD) is a neglected tropical disease for which novel and improved treatments are needed. The cysteine protease Cruzipain is one of the main targets for the development of novel drugs for the treatment of CD. Recent bioinformatics analyses have revealed four Cruzipain subtypes whose active sites differ in key positions for ligand recognition. These analyses suggest a possible effect on the substrate specificity and affinity for ligands. To better investigate the impact of substitutions in Cruzipain subtypes, we employed molecular dynamics simulations and varied structural analyses on representatives of each Cruzipain subtype. Our results indicated that the substitutions did not significantly affect the overall flexibility and conformation of these proteases. In contrast, we observed differences in their active site characteristics, including different electrostatic potentials, cavity volumes, and patterns of interactions with the virtual probes. The distinct alterations in the active site subsites, especially in the S2 subsite, suggest unique functional changes that could affect substrate binding, ligand recognition, and possibly enzymatic effectiveness in various biological situations. @article={003249663,author = {SANTOS, Lucianna Helene Silva; CAMPOS, Augusto César Broilo; SANTOS, Viviane Corrêa; FASSIO, Alexandre Victor; COSTA, Mauricio Garcia de Souza; FERREIRA, Rafaela Salgado.},title={Unraveling common patterns and differences among cruzipains through molecular dynamics simulations and structural analyses},journal={ACS Omega},note={v. 10, n. 18, p. 19115-19128},year={2025}}
@article={003249663,author = {SANTOS, Lucianna Helene Silva; CAMPOS, Augusto César Broilo; SANTOS, Viviane Corrêa; FASSIO, Alexandre Victor; COSTA, Mauricio Garcia de Souza; FERREIRA, Rafaela Salgado.},title={Unraveling common patterns and differences among cruzipains through molecular dynamics simulations and structural analyses},journal={ACS Omega},note={v. 10, n. 18, p. 19115-19128},year={2025}}