Pressure dependence of side chain 1H and 15N-chemical shifts in the model peptides Ac-Gly-Gly-Xxx-Ala-NH2.
ERLACH, Markus Beck; KOEHLER, Joerg; MUNTE, Claudia Elisabeth; KREMER, Werner; CRUSCA JUNIOR, Edson; KAINOSHO, Masatsune; KALBITZER, Hans Robert.
ERLACH, Markus Beck; KOEHLER, Joerg; MUNTE, Claudia Elisabeth; KREMER, Werner; CRUSCA JUNIOR, Edson; KAINOSHO, Masatsune; KALBITZER, Hans Robert.




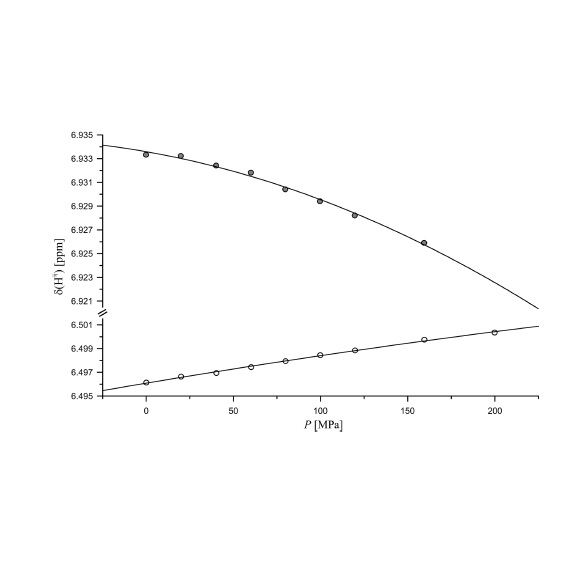 Abstract: For interpreting the pressure induced shifts of resonance lines of folded as well as unfolded proteins the availability of data from well-defined model systems is indispensable. Here, we report the pressure dependence of 1H and 15N chemical shifts of the side chain atoms in the protected tetrapeptides Ac-Gly-Gly-Xxx-Ala-NH2 (Xxx is one of the 20 canonical amino acids) measured at 800 MHz proton frequency. As observed earlier for other nuclei the chemical shifts of the side chain nuclei have a nonlinear dependence on pressure in the range from 0.1 to 200 MPa. The pressure response is described by a second degree polynomial with the pressure coefficients B1 and B2 that are dependent on the atom type and type of amino acid studied. A number of resonances could be assigned stereospecifically including the 1H and 15N resonances of the guanidine group of arginine. In addition, stereoselectively isotope labeled SAIL amino acids were used to support the stereochemical assignments. The random-coil pressure coefficients are also dependent on the neighbor in the sequence as an analysis of the data shows. For Ha and HN correction factors for different amino acids were derived. In addition, a simple correction of compression effects in thermodynamic analysis of structural transitions in proteins was derived on the basis of random-coil pressure coefficients.
Abstract: For interpreting the pressure induced shifts of resonance lines of folded as well as unfolded proteins the availability of data from well-defined model systems is indispensable. Here, we report the pressure dependence of 1H and 15N chemical shifts of the side chain atoms in the protected tetrapeptides Ac-Gly-Gly-Xxx-Ala-NH2 (Xxx is one of the 20 canonical amino acids) measured at 800 MHz proton frequency. As observed earlier for other nuclei the chemical shifts of the side chain nuclei have a nonlinear dependence on pressure in the range from 0.1 to 200 MPa. The pressure response is described by a second degree polynomial with the pressure coefficients B1 and B2 that are dependent on the atom type and type of amino acid studied. A number of resonances could be assigned stereospecifically including the 1H and 15N resonances of the guanidine group of arginine. In addition, stereoselectively isotope labeled SAIL amino acids were used to support the stereochemical assignments. The random-coil pressure coefficients are also dependent on the neighbor in the sequence as an analysis of the data shows. For Ha and HN correction factors for different amino acids were derived. In addition, a simple correction of compression effects in thermodynamic analysis of structural transitions in proteins was derived on the basis of random-coil pressure coefficients. @article={003006505,author = {ERLACH, Markus Beck; KOEHLER, Joerg; MUNTE, Claudia Elisabeth; KREMER, Werner; CRUSCA JUNIOR, Edson; KAINOSHO, Masatsune; KALBITZER, Hans Robert.},title={Pressure dependence of side chain 1H and 15N-chemical shifts in the model peptides Ac-Gly-Gly-Xxx-Ala-NH2},journal={Journal of Biomolecular NMR},note={v. 74, n. 8-9, p. 381-399},year={2020}}
@article={003006505,author = {ERLACH, Markus Beck; KOEHLER, Joerg; MUNTE, Claudia Elisabeth; KREMER, Werner; CRUSCA JUNIOR, Edson; KAINOSHO, Masatsune; KALBITZER, Hans Robert.},title={Pressure dependence of side chain 1H and 15N-chemical shifts in the model peptides Ac-Gly-Gly-Xxx-Ala-NH2},journal={Journal of Biomolecular NMR},note={v. 74, n. 8-9, p. 381-399},year={2020}}