Composition-structure-property correlations in rare-earth-doped heavy metal oxyfluoride glasses.
DOERENKAMP, Carsten; CARVAJAL, Eduar; MAGON, Cláudio José; FARIA, Walter José Gomes Juste; DONOSO, José Pedro; GOBATO, Y. Galvão; DE CAMARGO, Andrea Simone Stucchi; ECKERT, Hellmut.
DOERENKAMP, Carsten; CARVAJAL, Eduar; MAGON, Cláudio José; FARIA, Walter José Gomes Juste; DONOSO, José Pedro; GOBATO, Y. Galvão; DE CAMARGO, Andrea Simone Stucchi; ECKERT, Hellmut.
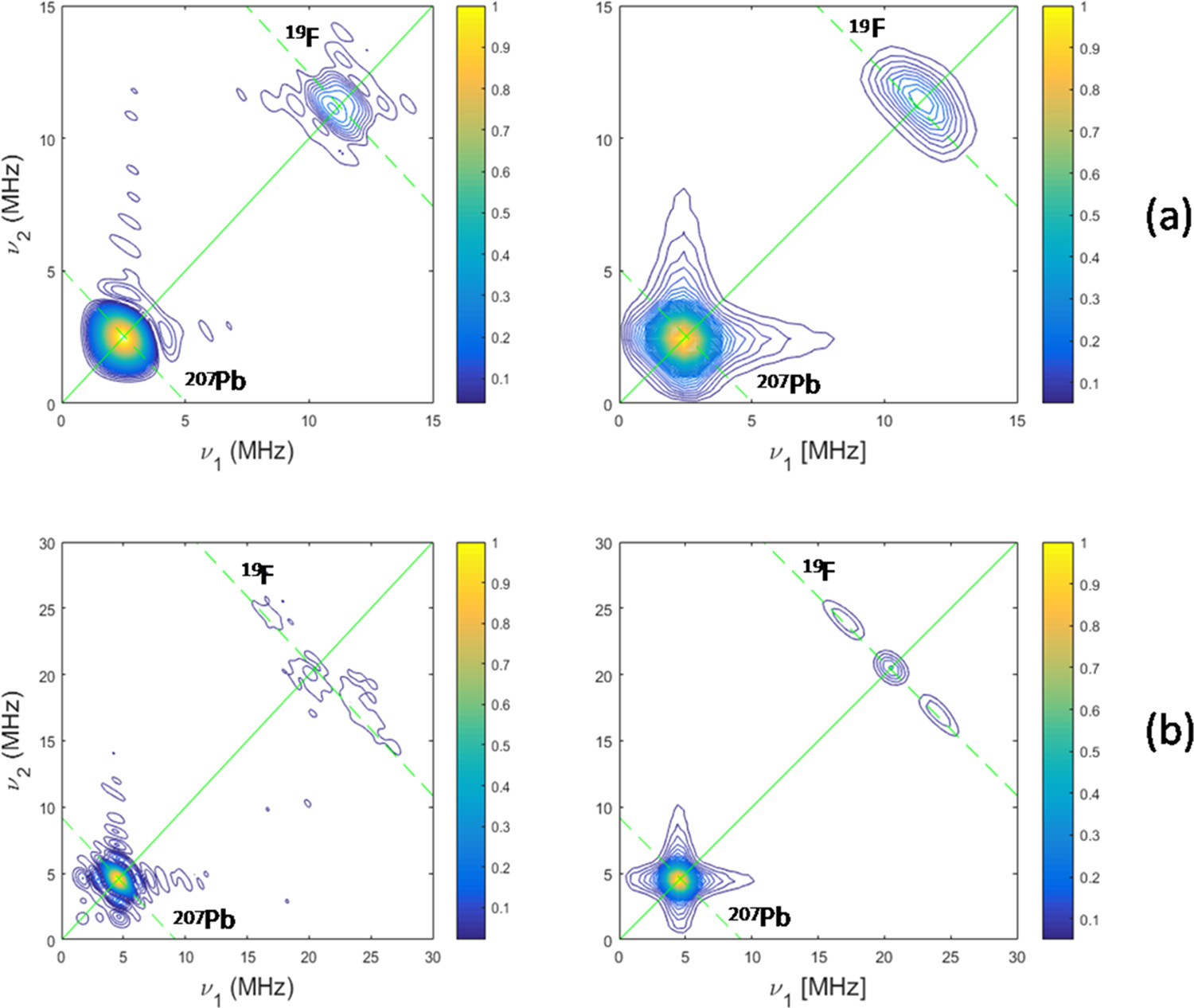 Abstract: Structure-property correlations in oxyfluoride glasses have been explored in a series of lead fluoroborate and lead fluorogermanate glasses with nominal compositions (50 - x - y - z)B2O3-40PbO-y(Al2O3)-(10 + x)PbF2-zREF3 and (50 - x - y)GeO2-40PbO-y(Al2O3)-(10 + x)PbF2-zREF3 (x, y = 0, 10, 0 = z = 0.5, RE = Eu, Yb). Starting from glasses with a fixed PbF2 content of 10 mol %, we explore the effects of (1) increasing PbF2 content to 20 mol % and (2) incorporating the intermediate oxide Al2O3 at the expense of GeO2 or B2O3. The emission characteristics studied on Eu-doped glasses are rationalized on the basis of structural information obtained by Raman, nuclear magnetic resonance (NMR), and pulsed electron paramagnetic resonance (EPR) spectroscopies on Yb-doped samples. In the Ge-oxyfluoride glasses, increasing PbF2 content results in enhanced excited-state lifetimes of the rare-earth ions, and for this system, Eu3+ emission profiles, NMR, and EPR results suggest an increased average number of fluoride ions in the first coordination sphere of the rare-earth ions. In contrast, the effect is much less apparent in the fluoroborate glasses. In both systems, Al2O3 incorporation results in pronounced changes in the fluorine speciation, indicating the formation of aluminum-fluorine bonds. Abstract: Structure-property correlations in oxyfluoride glasses have been explored in a series of lead fluoroborate and lead fluorogermanate glasses with nominal compositions (50 - x - y - z)B2O3-40PbO-y(Al2O3)-(10 + x)PbF2-zREF3 and (50 - x - y)GeO2-40PbO-y(Al2O3)-(10 + x)PbF2-zREF3 (x, y = 0, 10, 0 = z = 0.5, RE = Eu, Yb). Starting from glasses with a fixed PbF2 content of 10 mol %, we explore the effects of (1) increasing PbF2 content to 20 mol % and (2) incorporating the intermediate oxide Al2O3 at the expense of GeO2 or B2O3. The emission characteristics studied on Eu-doped glasses are rationalized on the basis of structural information obtained by Raman, nuclear magnetic resonance (NMR), and pulsed electron paramagnetic resonance (EPR) spectroscopies on Yb-doped samples. In the Ge-oxyfluoride glasses, increasing PbF2 content results in enhanced excited-state lifetimes of the rare-earth ions, and for this system, Eu3+ emission profiles, NMR, and EPR results suggest an increased average number of fluoride ions in the first coordination sphere of the rare-earth ions. In contrast, the effect is much less apparent in the fluoroborate glasses. In both systems, Al2O3 incorporation results in pronounced changes in the fluorine speciation, indicating the formation of aluminum-fluorine bonds. | |
| Journal of Physical Chemistry C |
| v. 123, n. 36, p. 22478-22490 - Ano: 2019 |
| Fator de Impacto: 4,309 |
| http://dx.doi.org/10.1021/acs.jpcc.9b05531 |  @article={002965351,author = {DOERENKAMP, Carsten; CARVAJAL, Eduar; MAGON, Cláudio José; FARIA, Walter José Gomes Juste; DONOSO, José Pedro; GOBATO, Y. Galvão; DE CAMARGO, Andrea Simone Stucchi; ECKERT, Hellmut.},title={Composition-structure-property correlations in rare-earth-doped heavy metal oxyfluoride glasses},journal={Journal of Physical Chemistry C},note={v. 123, n. 36, p. 22478-22490},year={2019}} @article={002965351,author = {DOERENKAMP, Carsten; CARVAJAL, Eduar; MAGON, Cláudio José; FARIA, Walter José Gomes Juste; DONOSO, José Pedro; GOBATO, Y. Galvão; DE CAMARGO, Andrea Simone Stucchi; ECKERT, Hellmut.},title={Composition-structure-property correlations in rare-earth-doped heavy metal oxyfluoride glasses},journal={Journal of Physical Chemistry C},note={v. 123, n. 36, p. 22478-22490},year={2019}} |



