Thermal behavior of inosine 5'-monophosphate complexes with cobalt(II), nickel(II), copper(II) and cadmium(II) cations.
JESUS, Jany Hellen Ferreira de; SOUZA, Matheus da Silva; ELLENA, Javier; CAVALHEIRO, Eder Tadeu Gomes.
JESUS, Jany Hellen Ferreira de; SOUZA, Matheus da Silva; ELLENA, Javier; CAVALHEIRO, Eder Tadeu Gomes.
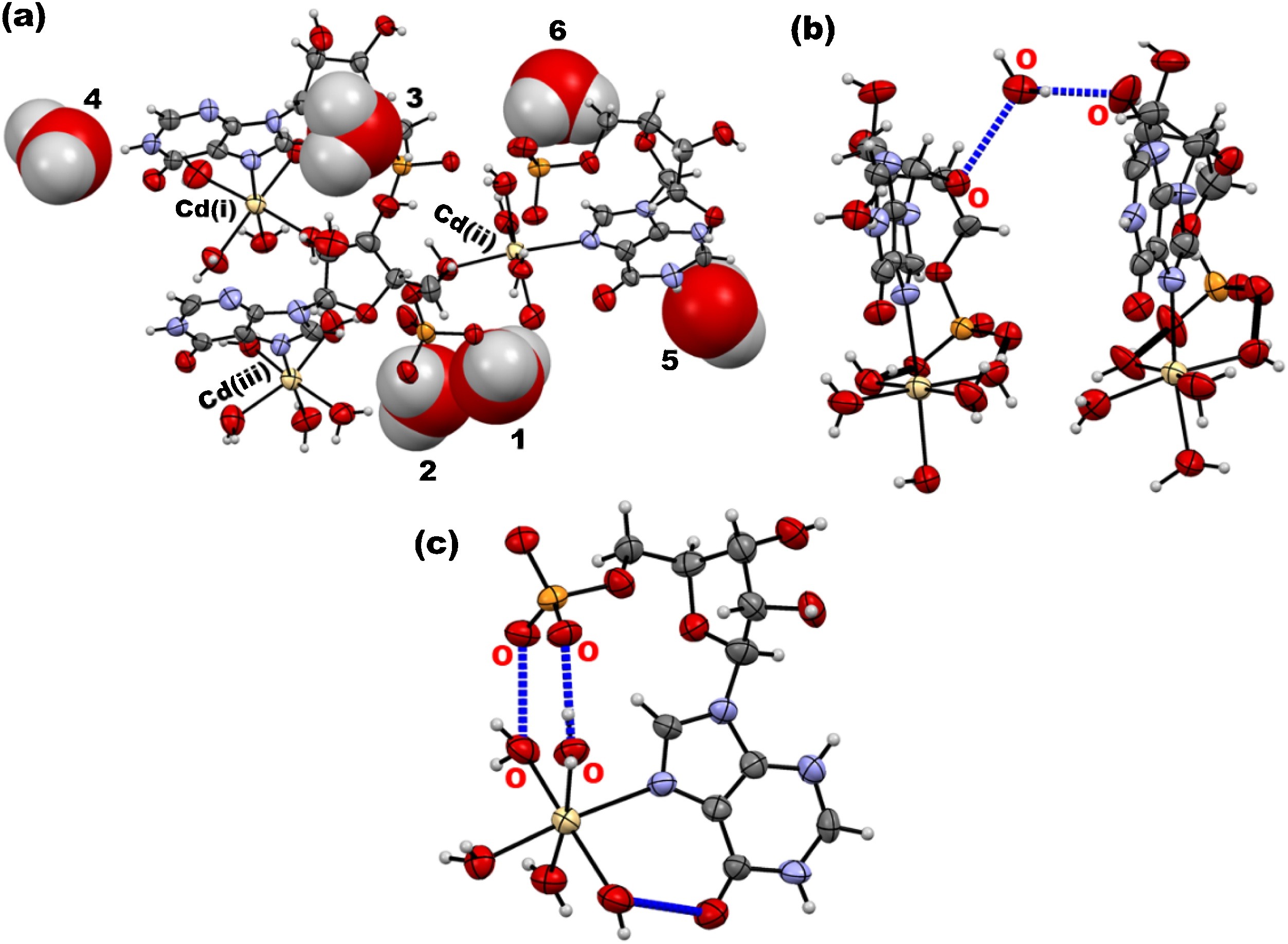 Abstract: Interaction of nucleotides and metals has been largely studied since they have biological importance. Divalent complexes of inosine 5'-monophosphate with cobalt(II) (IMP-Co), nickel(II) (IMP-Ni), copper(II) (IMP-Cu) and cadmium(II) (IMP-Cd) have been characterized by elemental analysis and submitted to thermogravimetry (TG), differential thermal analysis (DTA), differential scanning calorimetry (DSC) and thermogravimetry coupled to infrared spectroscopy (TG-FTIR). Cadmium(II) complex with inosine 5'-monophosphate had also its crystalline structure elucidated by single-crystal X-ray diffraction. Elemental analysis revealed that the isolated metal complexes presented a 1:1 stoichiometry. The thermal stability order was: IMP-Co (193?°C) = IMP-Cd (193°C) < IMP-Ni (206°C) < IMP-Cu (219?°C), after dehydration. Cd2+ ions present octahedral coordination with an IMP ligand through nitrogen donor atom and the remaining coordinating sites through five water molecules. The crystal packing assembly has a similar distribution to the DNA molecule, with alternation between the atoms leading to a helical conformation. Abstract: Interaction of nucleotides and metals has been largely studied since they have biological importance. Divalent complexes of inosine 5'-monophosphate with cobalt(II) (IMP-Co), nickel(II) (IMP-Ni), copper(II) (IMP-Cu) and cadmium(II) (IMP-Cd) have been characterized by elemental analysis and submitted to thermogravimetry (TG), differential thermal analysis (DTA), differential scanning calorimetry (DSC) and thermogravimetry coupled to infrared spectroscopy (TG-FTIR). Cadmium(II) complex with inosine 5'-monophosphate had also its crystalline structure elucidated by single-crystal X-ray diffraction. Elemental analysis revealed that the isolated metal complexes presented a 1:1 stoichiometry. The thermal stability order was: IMP-Co (193?°C) = IMP-Cd (193°C) < IMP-Ni (206°C) < IMP-Cu (219?°C), after dehydration. Cd2+ ions present octahedral coordination with an IMP ligand through nitrogen donor atom and the remaining coordinating sites through five water molecules. The crystal packing assembly has a similar distribution to the DNA molecule, with alternation between the atoms leading to a helical conformation. | |
| Thermochimica Acta |
| v.680, p. 178378-1-178378-7 - Ano: 2019 |
| Fator de Impacto: 2,251 |
| https://doi.org/10.1016/j.tca.2019.178378 |  @article={002958854,author = {JESUS, Jany Hellen Ferreira de; SOUZA, Matheus da Silva; ELLENA, Javier; CAVALHEIRO, Eder Tadeu Gomes.},title={Thermal behavior of inosine 5'-monophosphate complexes with cobalt(II), nickel(II), copper(II) and cadmium(II) cations},journal={Thermochimica Acta},note={v.680, p. 178378-1-178378-7},year={2019}} @article={002958854,author = {JESUS, Jany Hellen Ferreira de; SOUZA, Matheus da Silva; ELLENA, Javier; CAVALHEIRO, Eder Tadeu Gomes.},title={Thermal behavior of inosine 5'-monophosphate complexes with cobalt(II), nickel(II), copper(II) and cadmium(II) cations},journal={Thermochimica Acta},note={v.680, p. 178378-1-178378-7},year={2019}} |



