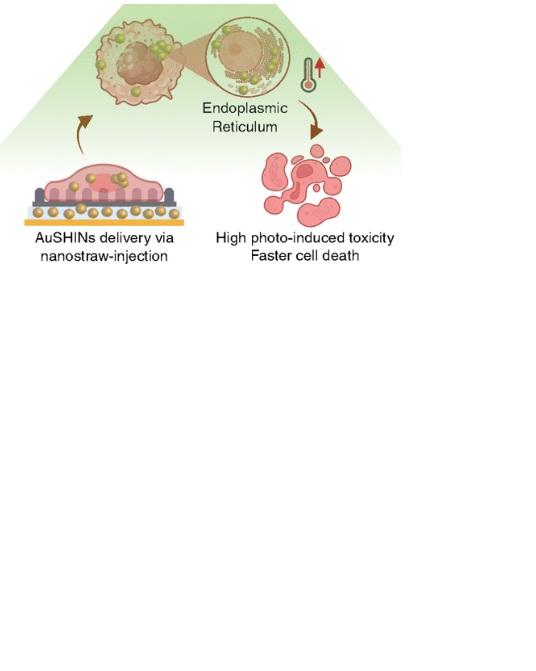
 Abstract: Gold shell-isolated nanoparticles (AuSHINs) are promising photothermal therapy (PTT) agents for cancer treatment due to their excellent photoconversion efficiency, biocompatibility, colloidal stability, and tunable properties, including size, shape, and surface functionalization. However, their therapeutic efficacy in in vitro assays is often limited by poor cellular uptake and lysosomal entrapment, which can result in nanoparticle degradation and a reduction in PTT effectiveness. In this study, we demonstrate that nanostraw-assisted injection enhances the PTT efficacy of AuSHINs compared to the conventional incubation method, as evaluated in human breast cancer cell lines: adenocarcinoma cells (MDA-MB-231) and glandular carcinoma cells (MCF7). This enhancement is attributed to three differences between the delivery methods: nanoparticle internalization, intracellular targeting, and the progression of cell death pathways. Nanostraw injection resulted in approximately 10-fold higher internalization of AuSHINs compared to 0.5-h incubation. Confocal fluorescence microscopy revealed that AuSHINs delivered via conventional incubation predominantly localize within lysosomes, whereas those introduced through nanostraw-assisted injection primarily targeted the endoplasmic reticulum (ER), thus avoiding lysosomal degradation. This differential targeting led to approximately a 2-fold higher reduction in the viability of photoactivated breast cancer cells treated with nanostraw-delivered AuSHINs. Furthermore, nanostraw-assisted injection accelerated the initiation of apoptosis relative to incubation. PTT-induced cell death was more pronounced in MCF7 cells compared to MDA-MB-231 cells, reflecting the higher resistance to therapy of the latter. These findings highlight the potential of nanostraw-assisted injection to enhance PTT, and we now face the challenge of integrating it into in vivo delivery strategies. Abstract: Gold shell-isolated nanoparticles (AuSHINs) are promising photothermal therapy (PTT) agents for cancer treatment due to their excellent photoconversion efficiency, biocompatibility, colloidal stability, and tunable properties, including size, shape, and surface functionalization. However, their therapeutic efficacy in in vitro assays is often limited by poor cellular uptake and lysosomal entrapment, which can result in nanoparticle degradation and a reduction in PTT effectiveness. In this study, we demonstrate that nanostraw-assisted injection enhances the PTT efficacy of AuSHINs compared to the conventional incubation method, as evaluated in human breast cancer cell lines: adenocarcinoma cells (MDA-MB-231) and glandular carcinoma cells (MCF7). This enhancement is attributed to three differences between the delivery methods: nanoparticle internalization, intracellular targeting, and the progression of cell death pathways. Nanostraw injection resulted in approximately 10-fold higher internalization of AuSHINs compared to 0.5-h incubation. Confocal fluorescence microscopy revealed that AuSHINs delivered via conventional incubation predominantly localize within lysosomes, whereas those introduced through nanostraw-assisted injection primarily targeted the endoplasmic reticulum (ER), thus avoiding lysosomal degradation. This differential targeting led to approximately a 2-fold higher reduction in the viability of photoactivated breast cancer cells treated with nanostraw-delivered AuSHINs. Furthermore, nanostraw-assisted injection accelerated the initiation of apoptosis relative to incubation. PTT-induced cell death was more pronounced in MCF7 cells compared to MDA-MB-231 cells, reflecting the higher resistance to therapy of the latter. These findings highlight the potential of nanostraw-assisted injection to enhance PTT, and we now face the challenge of integrating it into in vivo delivery strategies. |





 Abstract: Gold shell-isolated nanoparticles (AuSHINs) are promising photothermal therapy (PTT) agents for cancer treatment due to their excellent photoconversion efficiency, biocompatibility, colloidal stability, and tunable properties, including size, shape, and surface functionalization. However, their therapeutic efficacy in in vitro assays is often limited by poor cellular uptake and lysosomal entrapment, which can result in nanoparticle degradation and a reduction in PTT effectiveness. In this study, we demonstrate that nanostraw-assisted injection enhances the PTT efficacy of AuSHINs compared to the conventional incubation method, as evaluated in human breast cancer cell lines: adenocarcinoma cells (MDA-MB-231) and glandular carcinoma cells (MCF7). This enhancement is attributed to three differences between the delivery methods: nanoparticle internalization, intracellular targeting, and the progression of cell death pathways. Nanostraw injection resulted in approximately 10-fold higher internalization of AuSHINs compared to 0.5-h incubation. Confocal fluorescence microscopy revealed that AuSHINs delivered via conventional incubation predominantly localize within lysosomes, whereas those introduced through nanostraw-assisted injection primarily targeted the endoplasmic reticulum (ER), thus avoiding lysosomal degradation. This differential targeting led to approximately a 2-fold higher reduction in the viability of photoactivated breast cancer cells treated with nanostraw-delivered AuSHINs. Furthermore, nanostraw-assisted injection accelerated the initiation of apoptosis relative to incubation. PTT-induced cell death was more pronounced in MCF7 cells compared to MDA-MB-231 cells, reflecting the higher resistance to therapy of the latter. These findings highlight the potential of nanostraw-assisted injection to enhance PTT, and we now face the challenge of integrating it into in vivo delivery strategies.
Abstract: Gold shell-isolated nanoparticles (AuSHINs) are promising photothermal therapy (PTT) agents for cancer treatment due to their excellent photoconversion efficiency, biocompatibility, colloidal stability, and tunable properties, including size, shape, and surface functionalization. However, their therapeutic efficacy in in vitro assays is often limited by poor cellular uptake and lysosomal entrapment, which can result in nanoparticle degradation and a reduction in PTT effectiveness. In this study, we demonstrate that nanostraw-assisted injection enhances the PTT efficacy of AuSHINs compared to the conventional incubation method, as evaluated in human breast cancer cell lines: adenocarcinoma cells (MDA-MB-231) and glandular carcinoma cells (MCF7). This enhancement is attributed to three differences between the delivery methods: nanoparticle internalization, intracellular targeting, and the progression of cell death pathways. Nanostraw injection resulted in approximately 10-fold higher internalization of AuSHINs compared to 0.5-h incubation. Confocal fluorescence microscopy revealed that AuSHINs delivered via conventional incubation predominantly localize within lysosomes, whereas those introduced through nanostraw-assisted injection primarily targeted the endoplasmic reticulum (ER), thus avoiding lysosomal degradation. This differential targeting led to approximately a 2-fold higher reduction in the viability of photoactivated breast cancer cells treated with nanostraw-delivered AuSHINs. Furthermore, nanostraw-assisted injection accelerated the initiation of apoptosis relative to incubation. PTT-induced cell death was more pronounced in MCF7 cells compared to MDA-MB-231 cells, reflecting the higher resistance to therapy of the latter. These findings highlight the potential of nanostraw-assisted injection to enhance PTT, and we now face the challenge of integrating it into in vivo delivery strategies. @article={003249098,author = {CAMACHO, Sabrina Aléssio; AOKI, Pedro Henrique Benites; EKSTRAND, Frida; OLIVEIRA JUNIOR, Osvaldo Novais de; PRINZ, Christelle N.},title={Enhancing photothermal therapy against breast cancer cells by modulating the end point of gold shell-isolated nanoparticles using nanostraw-assisted injection},journal={ACS Applied Materials and Interfaces},note={v. 17, n. 19, p. 27816-27828},year={2025}}
@article={003249098,author = {CAMACHO, Sabrina Aléssio; AOKI, Pedro Henrique Benites; EKSTRAND, Frida; OLIVEIRA JUNIOR, Osvaldo Novais de; PRINZ, Christelle N.},title={Enhancing photothermal therapy against breast cancer cells by modulating the end point of gold shell-isolated nanoparticles using nanostraw-assisted injection},journal={ACS Applied Materials and Interfaces},note={v. 17, n. 19, p. 27816-27828},year={2025}}