Synthesis of ?1-pyrrolines via formal (3 + 2)-cycloaddition of 2H-azirines with enones promoted by visible light under continuous flow.
MARTELLI, Lorena Suelen Ribeiro; FURNIEL, Lucas Giani; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.
MARTELLI, Lorena Suelen Ribeiro; FURNIEL, Lucas Giani; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.





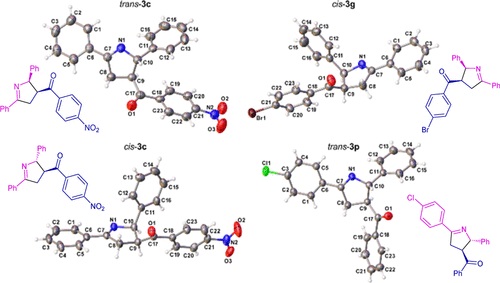 Abstract: This work reports the first synthesis of ?1-pyrrolines promoted by visible light under continuous flow, achieved through the formal (3 + 2)-cycloaddition of 2H-azirines to enones. A total of 22 examples of trisubstituted ?1-pyrrolines were prepared in only 30 min of residence time, with 41?93% yield and diastereomeric ratios up to 7:3. Furthermore, continuous flow conditions were also effective when chalcones were used as starting materials, leading to the formation of 7 tetrasubstituted pyrroles with an overall yield ranging from 12 to 47%, via a photocatalyzed cycloaddition-oxidation sequence.
Abstract: This work reports the first synthesis of ?1-pyrrolines promoted by visible light under continuous flow, achieved through the formal (3 + 2)-cycloaddition of 2H-azirines to enones. A total of 22 examples of trisubstituted ?1-pyrrolines were prepared in only 30 min of residence time, with 41?93% yield and diastereomeric ratios up to 7:3. Furthermore, continuous flow conditions were also effective when chalcones were used as starting materials, leading to the formation of 7 tetrasubstituted pyrroles with an overall yield ranging from 12 to 47%, via a photocatalyzed cycloaddition-oxidation sequence. @article={003246755,author = {MARTELLI, Lorena Suelen Ribeiro; FURNIEL, Lucas Giani; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.},title={Synthesis of ?1-pyrrolines via formal (3 + 2)-cycloaddition of 2H-azirines with enones promoted by visible light under continuous flow},journal={ACS Omega},note={v. 10, n. 17, p. 18017-18028 + supporting information},year={2025}}
@article={003246755,author = {MARTELLI, Lorena Suelen Ribeiro; FURNIEL, Lucas Giani; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.},title={Synthesis of ?1-pyrrolines via formal (3 + 2)-cycloaddition of 2H-azirines with enones promoted by visible light under continuous flow},journal={ACS Omega},note={v. 10, n. 17, p. 18017-18028 + supporting information},year={2025}}