Phylogenetic analysis and structural studies of heteromeric acetyl-CoA carboxylase from the oleaginous amazonian microalgae Ankistrodesmus sp.: insights into the BC and BCCP subunits.
MAVILA, Andry Mercedes; SANTILLAN, Jhon Antoni Vargas; MAMANI, Eloy Condori; FARRO, Erick Giancarlo Suclupe; FURTADO, Adriano Alves; SISLEY, Josué Manuel López; GONZALEZ, Silvia Lucila; PEREIRA, Humberto d'Muniz; DEL AGUILA, Jorge Luis Marapara; PAREDES, Roger Ruiz; COBOS, Marianela; GÓMEZ, Juan Carlos Castro; GARRATT, Richard Charles; CABREJOS, Diego Antonio Leonardo.
MAVILA, Andry Mercedes; SANTILLAN, Jhon Antoni Vargas; MAMANI, Eloy Condori; FARRO, Erick Giancarlo Suclupe; FURTADO, Adriano Alves; SISLEY, Josué Manuel López; GONZALEZ, Silvia Lucila; PEREIRA, Humberto d'Muniz; DEL AGUILA, Jorge Luis Marapara; PAREDES, Roger Ruiz; COBOS, Marianela; GÓMEZ, Juan Carlos Castro; GARRATT, Richard Charles; CABREJOS, Diego Antonio Leonardo.





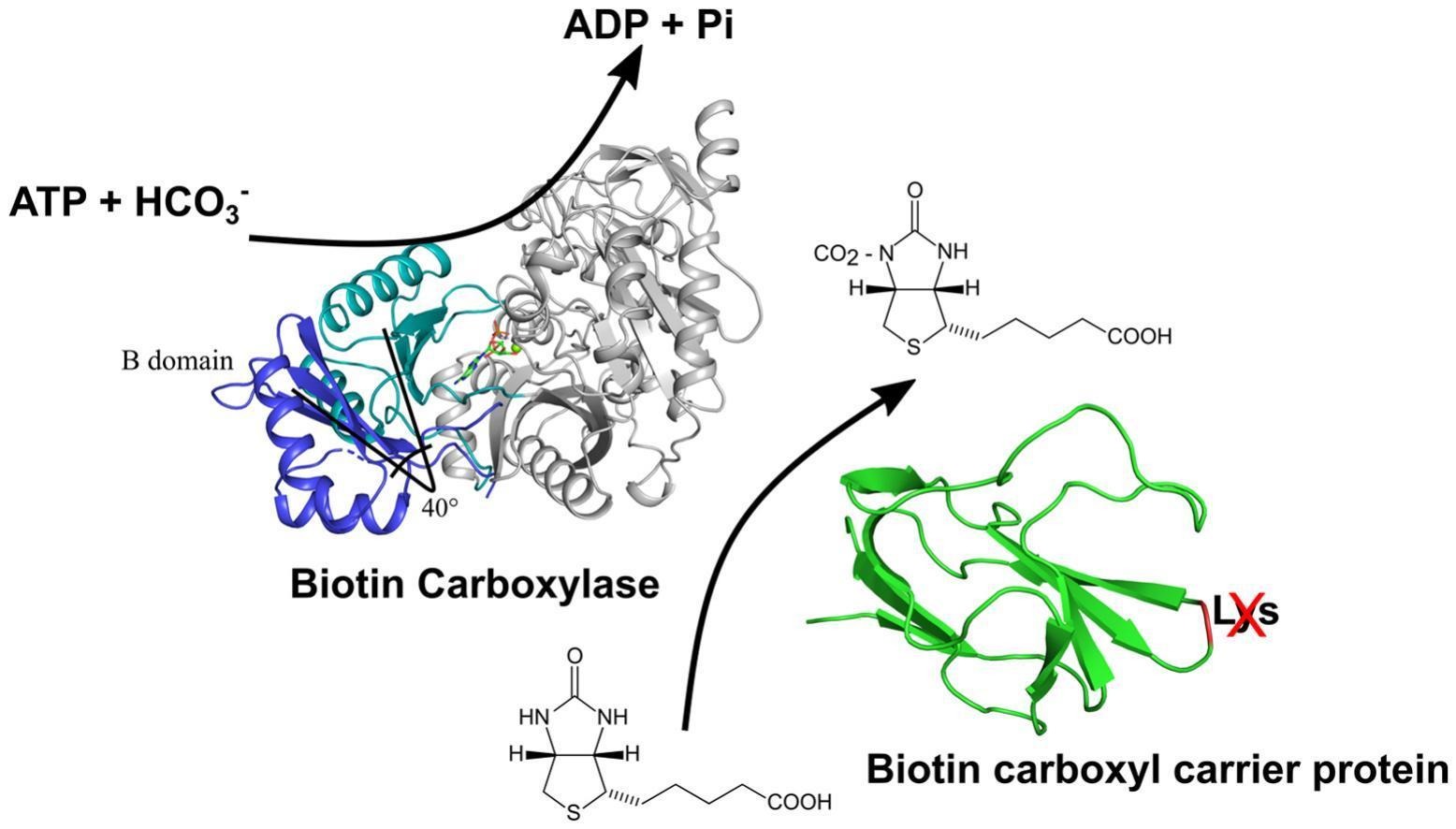 Abstract: Acetyl-CoA carboxylase (ACC) is an essential enzyme in fatty acid biosynthesis that catalyzes the formation of malonyl-CoA from acetyl-CoA. While structural studies on ACC components have largely focused on prokaryotes and higher plants, the assembly and molecular adaptations of ACC in microalgae remain underexplored. This study aimed to fill this gap by providing the first structural and evolutionary characterization of both biotin carboxylase (BC) and biotin carboxyl carrier protein (BCCP) from a microalga (Ankistrodesmus sp.). Phylogenetic analysis revealed distinct evolutionary trajectories for BC and BCCP, with BC forming a chlorophyte-specific clade closely related to other oleaginous species, while BCCP displayed two distinct isoforms within green algae, resulting from gene duplication. The crystallographic structure of BC was solved in its apo (1.75 Å) and ADP-Mg2+-bound (1.90 Å) states, revealing conserved conformational changes associated with cofactor binding. BCCP from Ankistrodesmus sp. displayed a unique QLGTF/H motif instead of the canonical AMKXM biotinylation motif, suggesting loss of biotinylation capacity. However, the presence of three additional lysines in the protruding thumb loop, with Lys95 as a candidate for biotin attachment, indicates potential compensatory adaptations. SEC-MALS and pull-down assays confirmed the formation of a stable 1:1 BC-BCCP complex, and circular dichroism showed increased thermal stability of the complex, supporting its structural stability. This study highlights unique structural adaptations in Ankistrodesmus sp. ACC, emphasizing the evolutionary plasticity of BC and BCCP. These insights provide a foundation for future investigations into ACC regulation in photosynthetic organisms and offer potential biotechnologic
Abstract: Acetyl-CoA carboxylase (ACC) is an essential enzyme in fatty acid biosynthesis that catalyzes the formation of malonyl-CoA from acetyl-CoA. While structural studies on ACC components have largely focused on prokaryotes and higher plants, the assembly and molecular adaptations of ACC in microalgae remain underexplored. This study aimed to fill this gap by providing the first structural and evolutionary characterization of both biotin carboxylase (BC) and biotin carboxyl carrier protein (BCCP) from a microalga (Ankistrodesmus sp.). Phylogenetic analysis revealed distinct evolutionary trajectories for BC and BCCP, with BC forming a chlorophyte-specific clade closely related to other oleaginous species, while BCCP displayed two distinct isoforms within green algae, resulting from gene duplication. The crystallographic structure of BC was solved in its apo (1.75 Å) and ADP-Mg2+-bound (1.90 Å) states, revealing conserved conformational changes associated with cofactor binding. BCCP from Ankistrodesmus sp. displayed a unique QLGTF/H motif instead of the canonical AMKXM biotinylation motif, suggesting loss of biotinylation capacity. However, the presence of three additional lysines in the protruding thumb loop, with Lys95 as a candidate for biotin attachment, indicates potential compensatory adaptations. SEC-MALS and pull-down assays confirmed the formation of a stable 1:1 BC-BCCP complex, and circular dichroism showed increased thermal stability of the complex, supporting its structural stability. This study highlights unique structural adaptations in Ankistrodesmus sp. ACC, emphasizing the evolutionary plasticity of BC and BCCP. These insights provide a foundation for future investigations into ACC regulation in photosynthetic organisms and offer potential biotechnologic @article={003243883,author = {MAVILA, Andry Mercedes; SANTILLAN, Jhon Antoni Vargas; MAMANI, Eloy Condori; FARRO, Erick Giancarlo Suclupe; FURTADO, Adriano Alves; SISLEY, Josué Manuel López; GONZALEZ, Silvia Lucila; PEREIRA, Humberto d'Muniz; DEL AGUILA, Jorge Luis Marapara; PAREDES, Roger Ruiz; COBOS, Marianela; GÓMEZ, Juan Carlos Castro; GARRATT, Richard Charles; CABREJOS, Diego Antonio Leonardo.},title={Phylogenetic analysis and structural studies of heteromeric acetyl-CoA carboxylase from the oleaginous amazonian microalgae Ankistrodesmus sp.: insights into the BC and BCCP subunits},journal={Journal of Structural Biology},note={v. 217, n. 2, p. 108200-1-108200-14 + supplementary data},year={2025}}
@article={003243883,author = {MAVILA, Andry Mercedes; SANTILLAN, Jhon Antoni Vargas; MAMANI, Eloy Condori; FARRO, Erick Giancarlo Suclupe; FURTADO, Adriano Alves; SISLEY, Josué Manuel López; GONZALEZ, Silvia Lucila; PEREIRA, Humberto d'Muniz; DEL AGUILA, Jorge Luis Marapara; PAREDES, Roger Ruiz; COBOS, Marianela; GÓMEZ, Juan Carlos Castro; GARRATT, Richard Charles; CABREJOS, Diego Antonio Leonardo.},title={Phylogenetic analysis and structural studies of heteromeric acetyl-CoA carboxylase from the oleaginous amazonian microalgae Ankistrodesmus sp.: insights into the BC and BCCP subunits},journal={Journal of Structural Biology},note={v. 217, n. 2, p. 108200-1-108200-14 + supplementary data},year={2025}}