In-depth mapping of DNA-PKcs signaling uncovers noncanonical features of its kinase specificity.
MARSHALL, Shannon; NAVARRO, Marcos Vicente de Albuquerque Salles; ASCEN?ÃO, Carolline Fernanda Rodrigues; DIBITETTO, Diego; SMOLKA, Marcus Bustamante.
MARSHALL, Shannon; NAVARRO, Marcos Vicente de Albuquerque Salles; ASCEN?ÃO, Carolline Fernanda Rodrigues; DIBITETTO, Diego; SMOLKA, Marcus Bustamante.





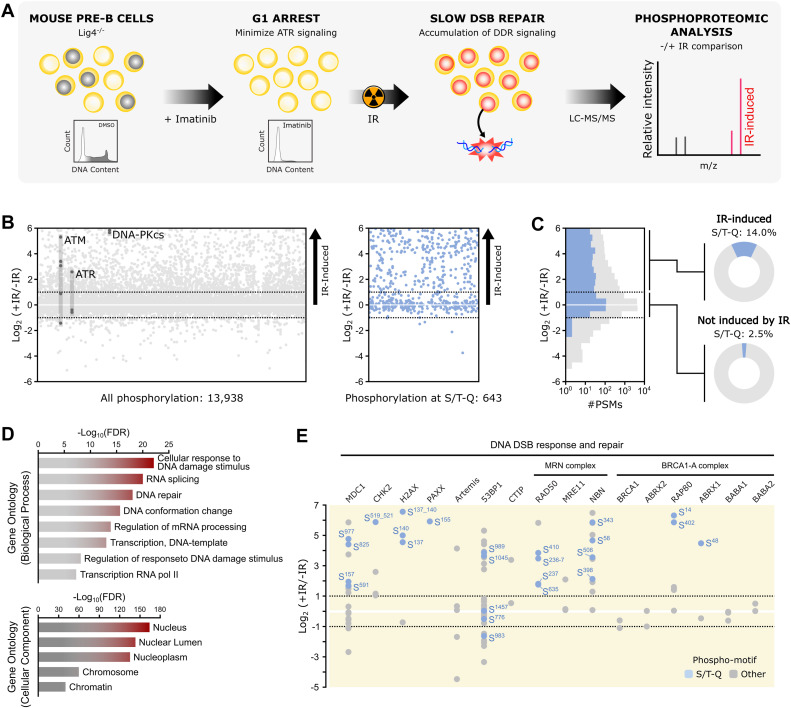 Abstract: DNA-PKcs is a DNA damage sensor kinase with established roles in DNA double-strand break repair via nonhomologous end joining. Recent studies have revealed additional roles of DNA-PKcs in the regulation of transcription, translation, and DNA replication. However, the substrates through which DNA-PKcs regulates these processes remain largely undefined. Here, we utilized quantitative phosphoproteomics to generate a high coverage map of DNA-PKcs signaling in response to ionizing radiation and mapped its interplay with the ATM kinase. Beyond the detection of the canonical S/T-Q phosphorylation motif, we uncovered a noncanonical mode of DNA-PKcs signaling targeting S/T-?-D/E motifs. Sequence and structural analyses of the DNA-PKcs substrate recognition pocket revealed unique features compared to closely related phosphatidylinositol 3-kinase-related kinases that may explain its broader substrate preference. These findings expand the repertoire of DNA-PKcs and ATM substrates while establishing a novel preferential phosphorylation motif for DNA-PKcs.
Abstract: DNA-PKcs is a DNA damage sensor kinase with established roles in DNA double-strand break repair via nonhomologous end joining. Recent studies have revealed additional roles of DNA-PKcs in the regulation of transcription, translation, and DNA replication. However, the substrates through which DNA-PKcs regulates these processes remain largely undefined. Here, we utilized quantitative phosphoproteomics to generate a high coverage map of DNA-PKcs signaling in response to ionizing radiation and mapped its interplay with the ATM kinase. Beyond the detection of the canonical S/T-Q phosphorylation motif, we uncovered a noncanonical mode of DNA-PKcs signaling targeting S/T-?-D/E motifs. Sequence and structural analyses of the DNA-PKcs substrate recognition pocket revealed unique features compared to closely related phosphatidylinositol 3-kinase-related kinases that may explain its broader substrate preference. These findings expand the repertoire of DNA-PKcs and ATM substrates while establishing a novel preferential phosphorylation motif for DNA-PKcs. @article={003236924,author = {MARSHALL, Shannon; NAVARRO, Marcos Vicente de Albuquerque Salles; ASCEN?ÃO, Carolline Fernanda Rodrigues; DIBITETTO, Diego; SMOLKA, Marcus Bustamante.},title={In-depth mapping of DNA-PKcs signaling uncovers noncanonical features of its kinase specificity},journal={Journal of Biological Chemistry},note={v. 300, n. 8, p. 107513-1-107513-11},year={2024}}
@article={003236924,author = {MARSHALL, Shannon; NAVARRO, Marcos Vicente de Albuquerque Salles; ASCEN?ÃO, Carolline Fernanda Rodrigues; DIBITETTO, Diego; SMOLKA, Marcus Bustamante.},title={In-depth mapping of DNA-PKcs signaling uncovers noncanonical features of its kinase specificity},journal={Journal of Biological Chemistry},note={v. 300, n. 8, p. 107513-1-107513-11},year={2024}}