Diastereoselective synthesis of highly functionalized ?-lactams via ugi reaction/michael addition.
VIDAL, Hérika Danielle Almeida; NUNES, Paulo Sérgio Gonçalves; MARTINEZ, Alice Karoline de Almeida; JANUÁRIO, Marcelo Augusto Pereira; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.
VIDAL, Hérika Danielle Almeida; NUNES, Paulo Sérgio Gonçalves; MARTINEZ, Alice Karoline de Almeida; JANUÁRIO, Marcelo Augusto Pereira; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.





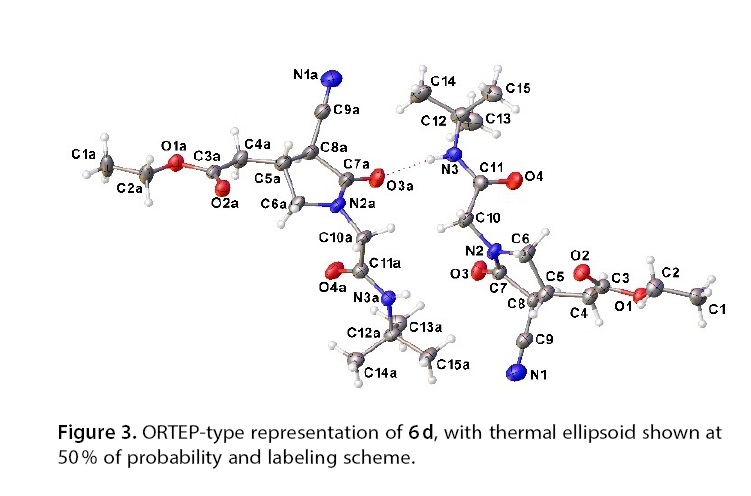 Abstract: The ?-lactam ring is a prominent feature in medicinal chemistry, and its synthesis has garnered significant interest due to its valuable properties. Among the ?-lactams, 2-oxopyrrolidine-3-carbonitrile derivatives stand out as versatile synthons that can be readily transformed into a variety of other functional groups. In this work, we successfully synthesized highly functionalized 3-cyano-2-pyrrolidinones with moderate to good overall yields using the Ugi reaction followed by intramolecular Michael addition. The process demonstrated excellent diastereoselectivity and showed good tolerance to a range of isonitriles and carbonyl compounds.
Abstract: The ?-lactam ring is a prominent feature in medicinal chemistry, and its synthesis has garnered significant interest due to its valuable properties. Among the ?-lactams, 2-oxopyrrolidine-3-carbonitrile derivatives stand out as versatile synthons that can be readily transformed into a variety of other functional groups. In this work, we successfully synthesized highly functionalized 3-cyano-2-pyrrolidinones with moderate to good overall yields using the Ugi reaction followed by intramolecular Michael addition. The process demonstrated excellent diastereoselectivity and showed good tolerance to a range of isonitriles and carbonyl compounds. @article={003228099,author = {VIDAL, Hérika Danielle Almeida; NUNES, Paulo Sérgio Gonçalves; MARTINEZ, Alice Karoline de Almeida; JANUÁRIO, Marcelo Augusto Pereira; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.},title={Diastereoselective synthesis of highly functionalized ?-lactams via ugi reaction/michael addition},journal={Chemistry: An Asian Journal},note={v. 20, n. 1, p. e202400917-1-e202400917-6 + supporting information},year={2025}}
@article={003228099,author = {VIDAL, Hérika Danielle Almeida; NUNES, Paulo Sérgio Gonçalves; MARTINEZ, Alice Karoline de Almeida; JANUÁRIO, Marcelo Augusto Pereira; SANTIAGO, Pedro Henrique de Oliveira; ELLENA, Javier; CORRÊA, Arlene Gonçalves.},title={Diastereoselective synthesis of highly functionalized ?-lactams via ugi reaction/michael addition},journal={Chemistry: An Asian Journal},note={v. 20, n. 1, p. e202400917-1-e202400917-6 + supporting information},year={2025}}