X-ray structure, chelation enhanced fluorescence effect, thermal and redox properties of a new europium(III) complex based on bioactive naphthoquinone.
GONÇALVES, Alice; CRUZ, Michele Macedo da; BISCOLI, Leandro de Oliveira; CABEZA, Natália Aparecida; FACCO, Janína Thomasi; BARROS, Herbert Lee Barbosa Veríssimo de; BOTERO, Eriton Rodrigo; HONORATO, João; ELLENA, Javier; FIORUCCI, Antonio Rogério; OLIVEIRA, Lincoln Carlos Silva de; STROPA, Jusinei Meireles; CASAGRANDE, Gleison Antônio; FAVARIN, Lis Regiane Vizolli; RODRIGUES, Daniela Cristina Manfroi; ANJOS, Ademir dos.
GONÇALVES, Alice; CRUZ, Michele Macedo da; BISCOLI, Leandro de Oliveira; CABEZA, Natália Aparecida; FACCO, Janína Thomasi; BARROS, Herbert Lee Barbosa Veríssimo de; BOTERO, Eriton Rodrigo; HONORATO, João; ELLENA, Javier; FIORUCCI, Antonio Rogério; OLIVEIRA, Lincoln Carlos Silva de; STROPA, Jusinei Meireles; CASAGRANDE, Gleison Antônio; FAVARIN, Lis Regiane Vizolli; RODRIGUES, Daniela Cristina Manfroi; ANJOS, Ademir dos.





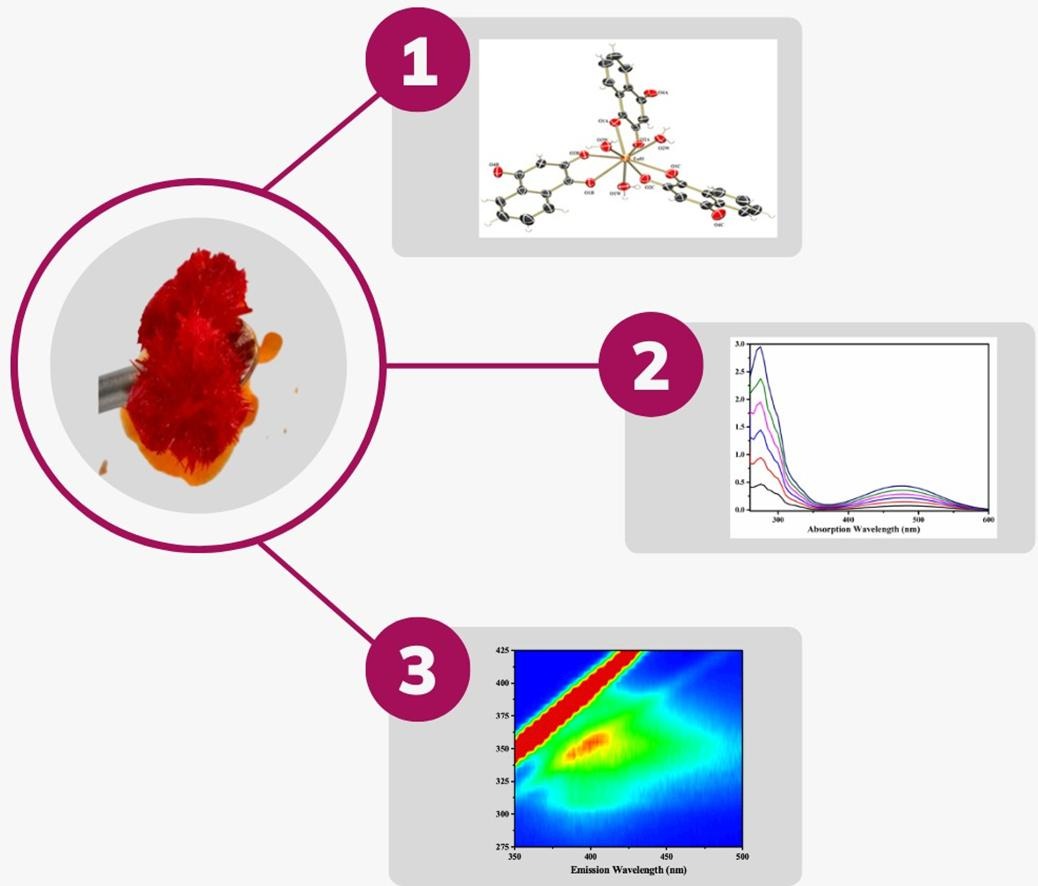 Abstract: The synthesis of metal complexes containing natural naphthoquinones has been highlighted due to their interesting electrochemical and spectroscopic properties, which may have a primordial influence on their pharmacological bioactivities. Simultaneously, processes of interaction of lanthanoid ions with organic ligands indicate the formation of coordination compounds that have marked luminescent properties of technological and medical diagnostic interest. Thus, this work describes the synthesis, characterization, and study of the properties of a new lawsone-EuIII metal complex, including the structural determination by XRD and the thermal, redox, and luminescent properties. The nine-coordinated complex exhibits a tri-hooded trigonal prismatic geometry, in which the metal ion is bound to nine oxygen atoms from three lawsone molecules and three water molecules, and the molecular structure still has approximately four water molecules as solvates, thus constituting [EuIII(C10H5O3)3(H2O)3].4H2O (FW = 797.49 g mol-1). The average Csingle bondO and Csingle bondC bond lengths indicate that naphthoquinone is coordinated in its anionic form, while the structural conformation shows intermolecular hydrogen interactions, which form interesting water channels. Thermal analyzes provided information regarding the decomposition behavior of the compound and its thermodynamic stability involving endothermic and exothermic processes. Electrochemical studies of the complex show that the processes associated with the ligand are shifted, with concomitant reduction of the Eu3+ ion. This new rearrangement is also responsible for differences in electronic transitions, such as the effect of increasing luminescence intensity (CHEF effect), and the phenomenon occurs through the antenna-like intramolecular interaction of the lawsonate anion with the europium(III) ion.
Abstract: The synthesis of metal complexes containing natural naphthoquinones has been highlighted due to their interesting electrochemical and spectroscopic properties, which may have a primordial influence on their pharmacological bioactivities. Simultaneously, processes of interaction of lanthanoid ions with organic ligands indicate the formation of coordination compounds that have marked luminescent properties of technological and medical diagnostic interest. Thus, this work describes the synthesis, characterization, and study of the properties of a new lawsone-EuIII metal complex, including the structural determination by XRD and the thermal, redox, and luminescent properties. The nine-coordinated complex exhibits a tri-hooded trigonal prismatic geometry, in which the metal ion is bound to nine oxygen atoms from three lawsone molecules and three water molecules, and the molecular structure still has approximately four water molecules as solvates, thus constituting [EuIII(C10H5O3)3(H2O)3].4H2O (FW = 797.49 g mol-1). The average Csingle bondO and Csingle bondC bond lengths indicate that naphthoquinone is coordinated in its anionic form, while the structural conformation shows intermolecular hydrogen interactions, which form interesting water channels. Thermal analyzes provided information regarding the decomposition behavior of the compound and its thermodynamic stability involving endothermic and exothermic processes. Electrochemical studies of the complex show that the processes associated with the ligand are shifted, with concomitant reduction of the Eu3+ ion. This new rearrangement is also responsible for differences in electronic transitions, such as the effect of increasing luminescence intensity (CHEF effect), and the phenomenon occurs through the antenna-like intramolecular interaction of the lawsonate anion with the europium(III) ion. @article={003157191,author = {GONÇALVES, Alice; CRUZ, Michele Macedo da; BISCOLI, Leandro de Oliveira; CABEZA, Natália Aparecida; FACCO, Janína Thomasi; BARROS, Herbert Lee Barbosa Veríssimo de; BOTERO, Eriton Rodrigo; HONORATO, João; ELLENA, Javier; FIORUCCI, Antonio Rogério; OLIVEIRA, Lincoln Carlos Silva de; STROPA, Jusinei Meireles; CASAGRANDE, Gleison Antônio; FAVARIN, Lis Regiane Vizolli; RODRIGUES, Daniela Cristina Manfroi; ANJOS, Ademir dos.},title={X-ray structure, chelation enhanced fluorescence effect, thermal and redox properties of a new europium(III) complex based on bioactive naphthoquinone},journal={Inorganica Chimica Acta},note={v. 558, p. 121758-1-121758-10 + supplementary data 1-2},year={2023}}
@article={003157191,author = {GONÇALVES, Alice; CRUZ, Michele Macedo da; BISCOLI, Leandro de Oliveira; CABEZA, Natália Aparecida; FACCO, Janína Thomasi; BARROS, Herbert Lee Barbosa Veríssimo de; BOTERO, Eriton Rodrigo; HONORATO, João; ELLENA, Javier; FIORUCCI, Antonio Rogério; OLIVEIRA, Lincoln Carlos Silva de; STROPA, Jusinei Meireles; CASAGRANDE, Gleison Antônio; FAVARIN, Lis Regiane Vizolli; RODRIGUES, Daniela Cristina Manfroi; ANJOS, Ademir dos.},title={X-ray structure, chelation enhanced fluorescence effect, thermal and redox properties of a new europium(III) complex based on bioactive naphthoquinone},journal={Inorganica Chimica Acta},note={v. 558, p. 121758-1-121758-10 + supplementary data 1-2},year={2023}}