Light-activated generation of nitric oxide (NO) and sulfite anion radicals (SO3?-) from a ruthenium(II) nitrosylsulphito complex.
ROVEDA JUNIOR, Antonio Carlos; SANTOS, Willy Glen; SOUZA, Maykon Lima; ADELSON, Charles N.; GONÇALVES, Felipe Souza; CASTELLANO, Eduardo Ernesto; GARINO, Claudio; FRANCO, Douglas Wagner; CARDOSO, Daniel Rodrigues.
ROVEDA JUNIOR, Antonio Carlos; SANTOS, Willy Glen; SOUZA, Maykon Lima; ADELSON, Charles N.; GONÇALVES, Felipe Souza; CASTELLANO, Eduardo Ernesto; GARINO, Claudio; FRANCO, Douglas Wagner; CARDOSO, Daniel Rodrigues.
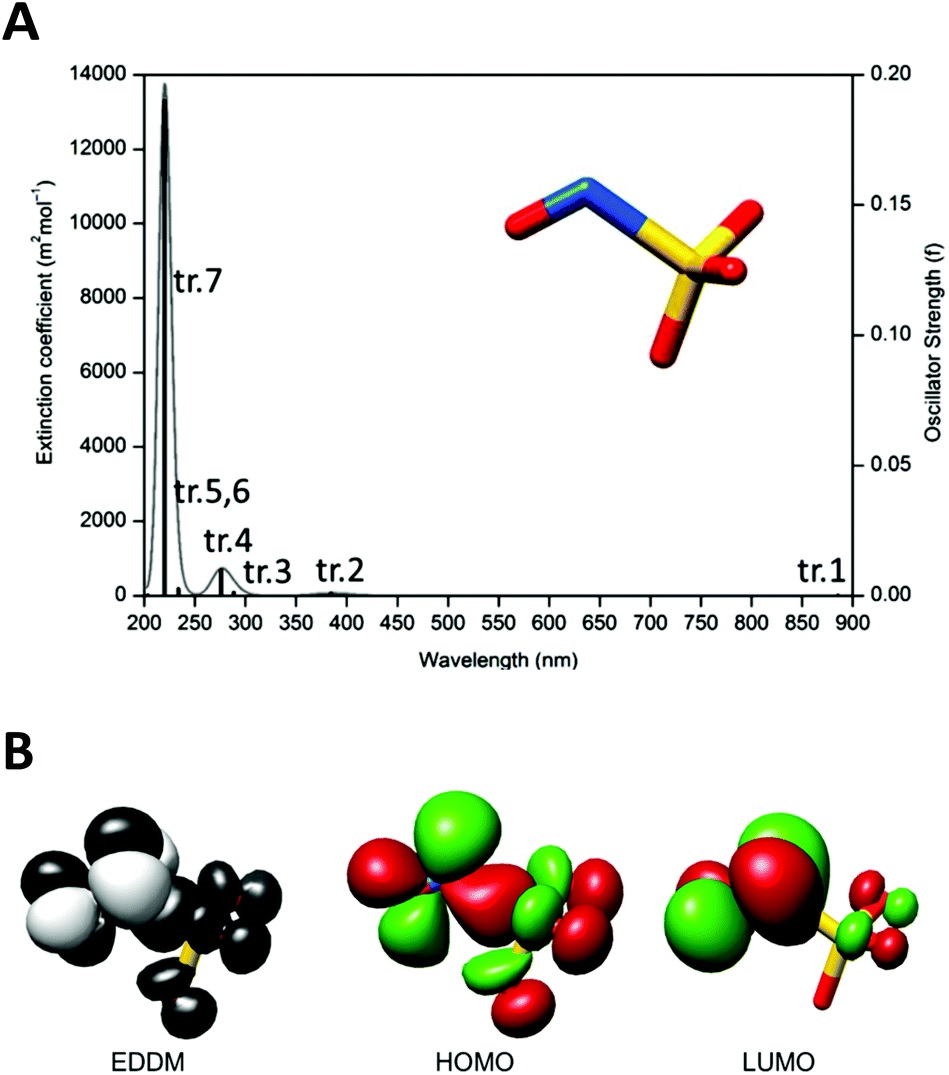 Abstract: This manuscript describes the preparation of a new Ru(II) nitrosylsulphito complex, trans-[Ru(NH3)4(isn) (N(O)SO3)]+ (complex 1), its spectroscopic and structural characterization, photochemistry, and thermal reactivity. Complex 1 was obtained by the reaction of sulfite ions (SO3 2-) with the nitrosyl complex trans- [Ru(NH3)4(isn)(NO)]3+ (complex 2) in aqueous solution resulting in the formation of the N-bonded nitrosylsulphito (N(O)SO3) ligand. To the best of our knowledge, only four nitrosylsulphito metal complexes have been described so far ( J. Chem. Soc., Dalton Trans., 1983, 2465-2472), and there is no information about the photochemistry of such complexes. Complex 1 was characterized by spectroscopic means (UV-Vis, EPR, FT-IR, 1H- and 15N-NMR), elemental analysis and single-crystal X-ray diffraction. The X-ray structure of the precursor complex 2 is also discussed in the manuscript and is used as a reference for comparisons with the structure of 1. Complex 1 is water-soluble and kinetically stable at pH 7.4, with a first-order rate constant of 3.1 × 10-5 s-1 for isn labilization at 298 K (t1/2 ~ 373 min). Under acidic conditions (1.0 M trifluoroacetic acid), 1 is stoichiometrically converted into the precursor complex 2. The reaction of hydroxide ions (OH-) with 1 and with 2 yields the Ru(II) nitro complex trans-[Ru(NH3)4(isn) (NO2)]+ with second-order rate constants of 2.1 and 10.5 M-1 s-1 (at 288 K), respectively, showing the nucleophilic attack of OH- at the nitrosyl in 2 (Ru?NO) and at the nitrosylsulphito in 1 (Ru?N(O)SO3). The pKa value of the -SO3 moiety of the N(O)SO3 ligand in 1 was determined to be 5.08 ± 0.06 (at 298 K). The unprecedented photochemistry of a nitrosylsulphito complex is investigated in detail with 1. The proposed mechanism is based on experimental (UV-Vis, EPR, NMR and Transient Absorption Laser Flash Photolysis) and theoretical data (DFT) and involves photorelease of the N(O)SO3 - ligand followed by formation of nitric oxide (NO?) and sulfite radicals (SO3 ?-, sulfur trioxide anion radical). Abstract: This manuscript describes the preparation of a new Ru(II) nitrosylsulphito complex, trans-[Ru(NH3)4(isn) (N(O)SO3)]+ (complex 1), its spectroscopic and structural characterization, photochemistry, and thermal reactivity. Complex 1 was obtained by the reaction of sulfite ions (SO3 2-) with the nitrosyl complex trans- [Ru(NH3)4(isn)(NO)]3+ (complex 2) in aqueous solution resulting in the formation of the N-bonded nitrosylsulphito (N(O)SO3) ligand. To the best of our knowledge, only four nitrosylsulphito metal complexes have been described so far ( J. Chem. Soc., Dalton Trans., 1983, 2465-2472), and there is no information about the photochemistry of such complexes. Complex 1 was characterized by spectroscopic means (UV-Vis, EPR, FT-IR, 1H- and 15N-NMR), elemental analysis and single-crystal X-ray diffraction. The X-ray structure of the precursor complex 2 is also discussed in the manuscript and is used as a reference for comparisons with the structure of 1. Complex 1 is water-soluble and kinetically stable at pH 7.4, with a first-order rate constant of 3.1 × 10-5 s-1 for isn labilization at 298 K (t1/2 ~ 373 min). Under acidic conditions (1.0 M trifluoroacetic acid), 1 is stoichiometrically converted into the precursor complex 2. The reaction of hydroxide ions (OH-) with 1 and with 2 yields the Ru(II) nitro complex trans-[Ru(NH3)4(isn) (NO2)]+ with second-order rate constants of 2.1 and 10.5 M-1 s-1 (at 288 K), respectively, showing the nucleophilic attack of OH- at the nitrosyl in 2 (Ru?NO) and at the nitrosylsulphito in 1 (Ru?N(O)SO3). The pKa value of the -SO3 moiety of the N(O)SO3 ligand in 1 was determined to be 5.08 ± 0.06 (at 298 K). The unprecedented photochemistry of a nitrosylsulphito complex is investigated in detail with 1. The proposed mechanism is based on experimental (UV-Vis, EPR, NMR and Transient Absorption Laser Flash Photolysis) and theoretical data (DFT) and involves photorelease of the N(O)SO3 - ligand followed by formation of nitric oxide (NO?) and sulfite radicals (SO3 ?-, sulfur trioxide anion radical). | |
| Dalton Transactions |
| v. 48, n.9, p. 10812-10823 - Ano: 2019 |
| Fator de Impacto: 4,052 |
| http://dx.doi.org/10.1039/c9dt01432b |  @article={002954676,author = {ROVEDA JUNIOR, Antonio Carlos; SANTOS, Willy Glen; SOUZA, Maykon Lima; ADELSON, Charles N.; GONÇALVES, Felipe Souza; CASTELLANO, Eduardo Ernesto; GARINO, Claudio; FRANCO, Douglas Wagner; CARDOSO, Daniel Rodrigues.},title={Light-activated generation of nitric oxide (NO) and sulfite anion radicals (SO3?-) from a ruthenium(II) nitrosylsulphito complex},journal={Dalton Transactions},note={v. 48, n.9, p. 10812-10823},year={2019}} @article={002954676,author = {ROVEDA JUNIOR, Antonio Carlos; SANTOS, Willy Glen; SOUZA, Maykon Lima; ADELSON, Charles N.; GONÇALVES, Felipe Souza; CASTELLANO, Eduardo Ernesto; GARINO, Claudio; FRANCO, Douglas Wagner; CARDOSO, Daniel Rodrigues.},title={Light-activated generation of nitric oxide (NO) and sulfite anion radicals (SO3?-) from a ruthenium(II) nitrosylsulphito complex},journal={Dalton Transactions},note={v. 48, n.9, p. 10812-10823},year={2019}} |




