Conformational dynamics of a G protein-coupled receptor helix 8 in lipid membranes.
DIJKMAN, Patricia M.; MUÑOZ-GARCÍA, Juan C.; LAVINGTON, Steven R.; KUMAGAI, Patricia Suemy; REIS, Rosana I. dos; YIN, Daniel; STANSFELD, Phillip J.; COSTA FILHO, Antonio José da; WATTS, Anthony.
DIJKMAN, Patricia M.; MUÑOZ-GARCÍA, Juan C.; LAVINGTON, Steven R.; KUMAGAI, Patricia Suemy; REIS, Rosana I. dos; YIN, Daniel; STANSFELD, Phillip J.; COSTA FILHO, Antonio José da; WATTS, Anthony.




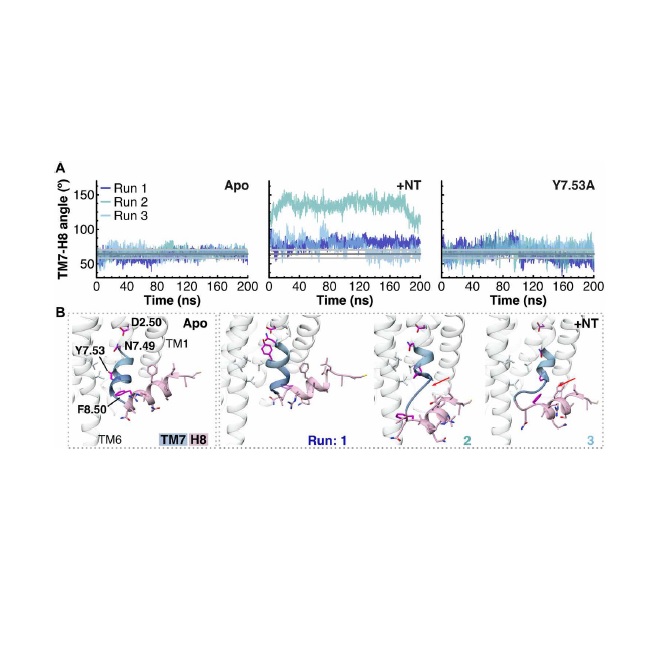 Abstract: G protein-coupled receptors (GPCRs) are the largest and pharmaceutically most important class of membrane proteins encoded in the human genome, characterized by a seven-transmembrane helix architecture and a C-terminal amphipathic helix 8 (H8). In a minority of GPCR structures solved to date, H8 either is absent or adopts an unusual conformation. The controversial existence of H8 of the class A GPCR neurotensin receptor 1 (NTS1) has been examined here for the nonthermostabilized receptor in a functionally supporting membrane environment using electron paramagnetic resonance, molecular dynamics simulations, and circular dichroism. Lipid-protein interactions with phosphatidylserine and phosphatidylethanolamine lipids, in particular, stabilize the residues 374 to 390 of NTS1 into forming a helix. Furthermore, introduction of a helix-breaking proline residue in H8 elicited an increase in ß-arrestin?NTS1 interactions observed in pull-down assays, suggesting that the structure and/or dynamics of H8 might play an important role in GPCR signaling.
Abstract: G protein-coupled receptors (GPCRs) are the largest and pharmaceutically most important class of membrane proteins encoded in the human genome, characterized by a seven-transmembrane helix architecture and a C-terminal amphipathic helix 8 (H8). In a minority of GPCR structures solved to date, H8 either is absent or adopts an unusual conformation. The controversial existence of H8 of the class A GPCR neurotensin receptor 1 (NTS1) has been examined here for the nonthermostabilized receptor in a functionally supporting membrane environment using electron paramagnetic resonance, molecular dynamics simulations, and circular dichroism. Lipid-protein interactions with phosphatidylserine and phosphatidylethanolamine lipids, in particular, stabilize the residues 374 to 390 of NTS1 into forming a helix. Furthermore, introduction of a helix-breaking proline residue in H8 elicited an increase in ß-arrestin?NTS1 interactions observed in pull-down assays, suggesting that the structure and/or dynamics of H8 might play an important role in GPCR signaling. @article={003004603,author = {DIJKMAN, Patricia M.; MUÑOZ-GARCÍA, Juan C.; LAVINGTON, Steven R.; KUMAGAI, Patricia Suemy; REIS, Rosana I. dos; YIN, Daniel; STANSFELD, Phillip J.; COSTA FILHO, Antonio José da; WATTS, Anthony.},title={Conformational dynamics of a G protein-coupled receptor helix 8 in lipid membranes},journal={Science Advances},note={v. 6, n. 33, p. 183173-1-183173-11},year={2020}}
@article={003004603,author = {DIJKMAN, Patricia M.; MUÑOZ-GARCÍA, Juan C.; LAVINGTON, Steven R.; KUMAGAI, Patricia Suemy; REIS, Rosana I. dos; YIN, Daniel; STANSFELD, Phillip J.; COSTA FILHO, Antonio José da; WATTS, Anthony.},title={Conformational dynamics of a G protein-coupled receptor helix 8 in lipid membranes},journal={Science Advances},note={v. 6, n. 33, p. 183173-1-183173-11},year={2020}}