Role of protein structure in variant annotation: structural insight of mutations causing 6-pyruvoyl-tetrahydropterin synthase deficiency.
MUNIZ, João Renato Carvalho; SZETO, Natalie Wing-Sum; FRISE, Rebecca; LEE, Wen Hwa; WANG, Xian-Song; THÖNY, Beat; HIMMELREICH, Nastassja; BLAU, Nenad; HSIAO, Kwang-Jen; LIU, Tze-Tze; GILEADI, Opher; OPPERMANN, Udo; VON DELFT, Frank; YUE, Wyatt W.; TANG, Nelson Leung-Sang.
MUNIZ, João Renato Carvalho; SZETO, Natalie Wing-Sum; FRISE, Rebecca; LEE, Wen Hwa; WANG, Xian-Song; THÖNY, Beat; HIMMELREICH, Nastassja; BLAU, Nenad; HSIAO, Kwang-Jen; LIU, Tze-Tze; GILEADI, Opher; OPPERMANN, Udo; VON DELFT, Frank; YUE, Wyatt W.; TANG, Nelson Leung-Sang.
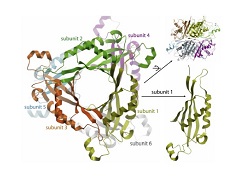 Abstract: Genetic defects on 6-pyruvoyl-tetrahydropterin synthase (PTPS) are the most prevalent cause of hyperphenylalaninaemia not due to phenylalanine hydrolyase deficiency (phenylketonuria). PTPS catalyses the second step of tetrahydrobiopterin (BH4) cofactor biosynthesis, and its deficiency represents the most common form of BH4 deficiency. Untreated PTPS deficiency results in depletion of the neurotransmitters dopamine, catecholamine and serotonin causing neurological symptoms. We archived reported missense variants of the PTS gene. Common in silico algorithms were used to predict the effects of such variants, and substantial proportions (up to 19%) of the variants were falsely classified as benign or uncertain. We have determined the crystal structure of the human PTPS hexamer, allowing another level of interpretation to understand the potential deleterious consequences of the variants from a structural perspective. The in silico and structure approaches appear to be complimentary and may provide new insights that are not available from each alone. Information from the protein structure suggested that the variants affecting amino acid residues required for interaction between monomeric subunits of the PTPS hexamer were those misclassified as benign by in silico algorithms. Our findings illustrate the important utility of 3D protein structure in interpretation of variants and also current limitations of in silico prediction algorithms. However, software to analyse mutation in the perspective of 3D protein structure is far less readily available than other in silico prediction tools. Abstract: Genetic defects on 6-pyruvoyl-tetrahydropterin synthase (PTPS) are the most prevalent cause of hyperphenylalaninaemia not due to phenylalanine hydrolyase deficiency (phenylketonuria). PTPS catalyses the second step of tetrahydrobiopterin (BH4) cofactor biosynthesis, and its deficiency represents the most common form of BH4 deficiency. Untreated PTPS deficiency results in depletion of the neurotransmitters dopamine, catecholamine and serotonin causing neurological symptoms. We archived reported missense variants of the PTS gene. Common in silico algorithms were used to predict the effects of such variants, and substantial proportions (up to 19%) of the variants were falsely classified as benign or uncertain. We have determined the crystal structure of the human PTPS hexamer, allowing another level of interpretation to understand the potential deleterious consequences of the variants from a structural perspective. The in silico and structure approaches appear to be complimentary and may provide new insights that are not available from each alone. Information from the protein structure suggested that the variants affecting amino acid residues required for interaction between monomeric subunits of the PTPS hexamer were those misclassified as benign by in silico algorithms. Our findings illustrate the important utility of 3D protein structure in interpretation of variants and also current limitations of in silico prediction algorithms. However, software to analyse mutation in the perspective of 3D protein structure is far less readily available than other in silico prediction tools. | |
| Pathology |
| v. 51, n. 3, p. 274-280 - Ano: 2019 |
| Fator de Impacto: 3,068 |
| http://dx.doi.org/10.1016/j.pathol.2018.11.011 |  @article={002935936,author = {MUNIZ, João Renato Carvalho; SZETO, Natalie Wing-Sum; FRISE, Rebecca; LEE, Wen Hwa; WANG, Xian-Song; THÖNY, Beat; HIMMELREICH, Nastassja; BLAU, Nenad; HSIAO, Kwang-Jen; LIU, Tze-Tze; GILEADI, Opher; OPPERMANN, Udo; VON DELFT, Frank; YUE, Wyatt W.; TANG, Nelson Leung-Sang.},title={Role of protein structure in variant annotation: structural insight of mutations causing 6-pyruvoyl-tetrahydropterin synthase deficiency},journal={Pathology},note={v. 51, n. 3, p. 274-280},year={2019}} @article={002935936,author = {MUNIZ, João Renato Carvalho; SZETO, Natalie Wing-Sum; FRISE, Rebecca; LEE, Wen Hwa; WANG, Xian-Song; THÖNY, Beat; HIMMELREICH, Nastassja; BLAU, Nenad; HSIAO, Kwang-Jen; LIU, Tze-Tze; GILEADI, Opher; OPPERMANN, Udo; VON DELFT, Frank; YUE, Wyatt W.; TANG, Nelson Leung-Sang.},title={Role of protein structure in variant annotation: structural insight of mutations causing 6-pyruvoyl-tetrahydropterin synthase deficiency},journal={Pathology},note={v. 51, n. 3, p. 274-280},year={2019}} |



