Biochemical characterization and low-resolution SAXS structure of two-domain endoglucanase BlCel9 from Bacillus licheniformis.
ARAÚJO, Evandro Ares de; OLIVEIRA NETO, Mário de; POLIKARPOV, Igor.
ARAÚJO, Evandro Ares de; OLIVEIRA NETO, Mário de; POLIKARPOV, Igor.
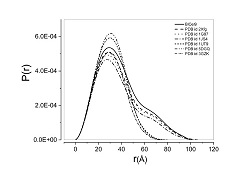 Abstract: Lignocellulose feedstock constitutes the most abundant carbon source in the biosphere; however, its recalcitrance remains a challenge for microbial conversion into biofuel and bioproducts. Bacillus licheniformis is a microbial mesophilic bacterium capable of secreting a large number of glycoside hydrolase (GH) enzymes, including a glycoside hydrolase from GH family 9 (BlCel9). Here, we conducted biochemical and biophysical studies of recombinant BlCel9, and its low-resolution molecular shape was retrieved from small angle X-ray scattering (SAXS) data. BlCel9 is an endoglucanase exhibiting maximum catalytic efficiency at pH 7.0 and 60 °C. Furthermore, it retains 80% of catalytic activity within a broad range of pH values (5.5-8.5) and temperatures (up to 50 °C) for extended periods of time (over 48 h). It exhibits the highest hydrolytic activity against phosphoric acid swollen cellulose (PASC), followed by bacterial cellulose (BC), filter paper (FP), and to a lesser extent carboxymethylcellulose (CMC). The HPAEC-PAD analysis of the hydrolytic products demonstrated that the end product of the enzymatic hydrolysis is primarily cellobiose, and also small amounts of glucose, cellotriose, and cellotetraose are produced. SAXS data analysis revealed that the enzyme adopts a monomeric state in solution and has a molecular mass of 65.8 kDa as estimated from SAXS data. The BlCel9 has an elongated shape composed of an N-terminal family 3 carbohydrate-binding module (CBM3c) and a C-terminal GH9 catalytic domain joined together by 20 amino acid residue long linker peptides. The domains are closely juxtaposed in an extended conformation and form a relatively rigid structure in solution, indicating that the interactions between the CBM3c and GH9 catalytic domains might play a key role in cooperative cellulose biomass recognition and hydrolysis. Abstract: Lignocellulose feedstock constitutes the most abundant carbon source in the biosphere; however, its recalcitrance remains a challenge for microbial conversion into biofuel and bioproducts. Bacillus licheniformis is a microbial mesophilic bacterium capable of secreting a large number of glycoside hydrolase (GH) enzymes, including a glycoside hydrolase from GH family 9 (BlCel9). Here, we conducted biochemical and biophysical studies of recombinant BlCel9, and its low-resolution molecular shape was retrieved from small angle X-ray scattering (SAXS) data. BlCel9 is an endoglucanase exhibiting maximum catalytic efficiency at pH 7.0 and 60 °C. Furthermore, it retains 80% of catalytic activity within a broad range of pH values (5.5-8.5) and temperatures (up to 50 °C) for extended periods of time (over 48 h). It exhibits the highest hydrolytic activity against phosphoric acid swollen cellulose (PASC), followed by bacterial cellulose (BC), filter paper (FP), and to a lesser extent carboxymethylcellulose (CMC). The HPAEC-PAD analysis of the hydrolytic products demonstrated that the end product of the enzymatic hydrolysis is primarily cellobiose, and also small amounts of glucose, cellotriose, and cellotetraose are produced. SAXS data analysis revealed that the enzyme adopts a monomeric state in solution and has a molecular mass of 65.8 kDa as estimated from SAXS data. The BlCel9 has an elongated shape composed of an N-terminal family 3 carbohydrate-binding module (CBM3c) and a C-terminal GH9 catalytic domain joined together by 20 amino acid residue long linker peptides. The domains are closely juxtaposed in an extended conformation and form a relatively rigid structure in solution, indicating that the interactions between the CBM3c and GH9 catalytic domains might play a key role in cooperative cellulose biomass recognition and hydrolysis. | |
| Applied Microbiology and Biotechnology |
| v. 103, n. 3, p. 1275-1285 - Ano: 2019 |
| Fator de Impacto: 3,340 |
| http://dx.doi.org/10.1007/s00253-018-9508-1 |  @article={002931232,author = {ARAÚJO, Evandro Ares de; OLIVEIRA NETO, Mário de; POLIKARPOV, Igor.},title={Biochemical characterization and low-resolution SAXS structure of two-domain endoglucanase BlCel9 from Bacillus licheniformis},journal={Applied Microbiology and Biotechnology},note={v. 103, n. 3, p. 1275-1285},year={2019}} @article={002931232,author = {ARAÚJO, Evandro Ares de; OLIVEIRA NETO, Mário de; POLIKARPOV, Igor.},title={Biochemical characterization and low-resolution SAXS structure of two-domain endoglucanase BlCel9 from Bacillus licheniformis},journal={Applied Microbiology and Biotechnology},note={v. 103, n. 3, p. 1275-1285},year={2019}} |



