The special role of B(C6F5)3 in the single electron reduction of quinones by radicals.
TAO, Xin; DANILIUC, Constantin G.; KNITSCH, Robert; HANSEN, Michael Ryan; ECKERT, Hellmut; LÜBBESMEYER, Maximilian; STUDER, Armido; KEHR, Gerald; ERKER, Gerhard.
TAO, Xin; DANILIUC, Constantin G.; KNITSCH, Robert; HANSEN, Michael Ryan; ECKERT, Hellmut; LÜBBESMEYER, Maximilian; STUDER, Armido; KEHR, Gerald; ERKER, Gerhard.
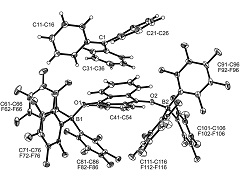 Abstract: In the presence of two molar equiv. of B(C6F5)3p-benzoquinone reacts with persistent radicals TEMPO, trityl or decamethylferrocene by single electron transfer to give doubly O-borylated benzosemiquinone radical anions with TEMPO+, trityl or Cp2*Fe+ ferrocenium counter cations. All three [(C6F5)3B]2-semiquinone radical anion salts were characterized by X-ray diffraction. The addition of donor reagent THF or DMSO induced rapid back electron transfer, in the case of the [(C6F5)3B]2-semiquinone radical anion oxoammonium salt giving rise to the formation of the (C6F5)3B-DMSO (or THF) Lewis adduct, p-benzoquinone and the TEMPO radical. The reaction of 9,10-anthraquinone or acenaphthenequinone with either the Gomberg dimer or Cp2*Fe in 1 : 1 stoichiometry in the presence of two molar equiv. of B(C6F5)3 gave the respective two-fold O-B(C6F5)3 containing 9,10-anthrasemiquinone or acenaphthene-semiquinone radical anion salts with either Ph3C+ or Cp2*Fe+ counter cations. These products were also characterized by X-ray diffraction. The Cp2*Fe+ salts showed analogous back electron shuttling behavior upon treatment with DMSO. 9,10-Phenanthrenequinone reacted analogously with B(C6F5)3 and the electron rich ferrocene. The Cp2*Fe+ [(C6F5)3B]2-9,10-phenanthrene-semiquinone salt was characterized by X-ray diffraction. The radical anions were characterized by ESR spectroscopy. Abstract: In the presence of two molar equiv. of B(C6F5)3p-benzoquinone reacts with persistent radicals TEMPO, trityl or decamethylferrocene by single electron transfer to give doubly O-borylated benzosemiquinone radical anions with TEMPO+, trityl or Cp2*Fe+ ferrocenium counter cations. All three [(C6F5)3B]2-semiquinone radical anion salts were characterized by X-ray diffraction. The addition of donor reagent THF or DMSO induced rapid back electron transfer, in the case of the [(C6F5)3B]2-semiquinone radical anion oxoammonium salt giving rise to the formation of the (C6F5)3B-DMSO (or THF) Lewis adduct, p-benzoquinone and the TEMPO radical. The reaction of 9,10-anthraquinone or acenaphthenequinone with either the Gomberg dimer or Cp2*Fe in 1 : 1 stoichiometry in the presence of two molar equiv. of B(C6F5)3 gave the respective two-fold O-B(C6F5)3 containing 9,10-anthrasemiquinone or acenaphthene-semiquinone radical anion salts with either Ph3C+ or Cp2*Fe+ counter cations. These products were also characterized by X-ray diffraction. The Cp2*Fe+ salts showed analogous back electron shuttling behavior upon treatment with DMSO. 9,10-Phenanthrenequinone reacted analogously with B(C6F5)3 and the electron rich ferrocene. The Cp2*Fe+ [(C6F5)3B]2-9,10-phenanthrene-semiquinone salt was characterized by X-ray diffraction. The radical anions were characterized by ESR spectroscopy. | |
| Chemical Science |
| v. 9, n. 41, p. 8011-8018 - Ano: 2018 |
| Fator de Impacto: 9,063 |
| http://dx.doi.org/10.1039/c8sc03005g |  @article={002912059,author = {TAO, Xin; DANILIUC, Constantin G.; KNITSCH, Robert; HANSEN, Michael Ryan; ECKERT, Hellmut; LÜBBESMEYER, Maximilian; STUDER, Armido; KEHR, Gerald; ERKER, Gerhard.},title={The special role of B(C6F5)3 in the single electron reduction of quinones by radicals},journal={Chemical Science},note={v. 9, n. 41, p. 8011-8018},year={2018}} @article={002912059,author = {TAO, Xin; DANILIUC, Constantin G.; KNITSCH, Robert; HANSEN, Michael Ryan; ECKERT, Hellmut; LÜBBESMEYER, Maximilian; STUDER, Armido; KEHR, Gerald; ERKER, Gerhard.},title={The special role of B(C6F5)3 in the single electron reduction of quinones by radicals},journal={Chemical Science},note={v. 9, n. 41, p. 8011-8018},year={2018}} |



