Structural and evolutionary analyses of PR-4 SUGARWINs points to a different pattern of protein function.
MAIA, Lorhenn Bryanda Lemes; PEREIRA, Humberto d'Muniz; GARRATT, Richard Charles; BRANDÃO-NETO, José; SILVA, Flavio Henrique; TOYAMA, Danyelle; DIAS, Renata O; BACHEGA, José Fernando Ruggiero; PEIXOTO, Julia Vasconcellos; SILVA-FILHO, Marcio de Castro.
MAIA, Lorhenn Bryanda Lemes; PEREIRA, Humberto d'Muniz; GARRATT, Richard Charles; BRANDÃO-NETO, José; SILVA, Flavio Henrique; TOYAMA, Danyelle; DIAS, Renata O; BACHEGA, José Fernando Ruggiero; PEIXOTO, Julia Vasconcellos; SILVA-FILHO, Marcio de Castro.





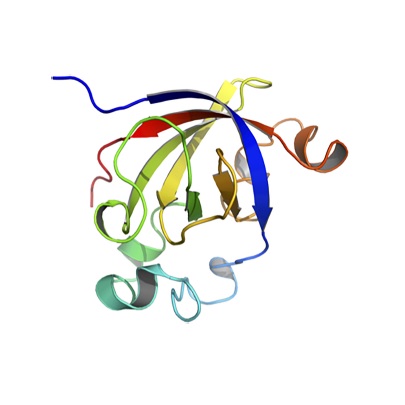 Abstract: SUGARWINs are PR-4 proteins associated with sugarcane defense against phytopathogens. Their expression is induced in response to damage by Diatraea saccharalis larvae. These proteins play an important role in plant defense, in particular against fungal pathogens, such as Colletothricum falcatum (Went) and Fusarium verticillioides. The pathogenesis-related protein-4 (PR-4) family is a group of proteins equipped with a BARWIN domain, which may be associated with a chitin-binding domain also known as the hevein-like domain. Several PR-4 proteins exhibit both chitinase and RNase activity, with the latter being associated with the presence of two histidine residues H11 and H113 (BARWIN) [H44 and H146, SUGARWINs] in the BARWIN-like domain. In sugarcane, similar to other PR-4 proteins, SUGARWIN1 exhibits ribonuclease, chitosanase and chitinase activities, whereas SUGARWIN2 only exhibits chitosanase activity. In order to decipher the structural determinants involved in this diverse range of enzyme specificities, we determined the 3-D structure of SUGARWIN2, at 1.55Å by X-ray diffraction. This is the first structure of a PR-4 protein where the first histidine has been replaced by asparagine and was subsequently used to build a homology model for SUGARWIN1. Molecular dynamics simulations of both proteins revealed the presence of a flexible loop only in SUGARWIN1 and we postulate that this, together with the presence of the catalytic histidine at position 42, renders it competent as a ribonuclease. The more electropositive surface potential of SUGARWIN1 would also be expected to favor complex formation with RNA. A phylogenetic analysis of PR-4 proteins obtained from 106 Embryophyta genomes showed that both catalytic histidines are widespread among them with few replacements in these amino acid positions during the gene family evolutionary history. We observe that the H11 replacement by N11 is also present in two other sugarcane PR-4 proteins: SUGARWIN3 and SUGARWIN4. We propose that RNase activity was present in the first Embryophyta PR-4 proteins but was recently lost in members of this family during the course of evolution
Abstract: SUGARWINs are PR-4 proteins associated with sugarcane defense against phytopathogens. Their expression is induced in response to damage by Diatraea saccharalis larvae. These proteins play an important role in plant defense, in particular against fungal pathogens, such as Colletothricum falcatum (Went) and Fusarium verticillioides. The pathogenesis-related protein-4 (PR-4) family is a group of proteins equipped with a BARWIN domain, which may be associated with a chitin-binding domain also known as the hevein-like domain. Several PR-4 proteins exhibit both chitinase and RNase activity, with the latter being associated with the presence of two histidine residues H11 and H113 (BARWIN) [H44 and H146, SUGARWINs] in the BARWIN-like domain. In sugarcane, similar to other PR-4 proteins, SUGARWIN1 exhibits ribonuclease, chitosanase and chitinase activities, whereas SUGARWIN2 only exhibits chitosanase activity. In order to decipher the structural determinants involved in this diverse range of enzyme specificities, we determined the 3-D structure of SUGARWIN2, at 1.55Å by X-ray diffraction. This is the first structure of a PR-4 protein where the first histidine has been replaced by asparagine and was subsequently used to build a homology model for SUGARWIN1. Molecular dynamics simulations of both proteins revealed the presence of a flexible loop only in SUGARWIN1 and we postulate that this, together with the presence of the catalytic histidine at position 42, renders it competent as a ribonuclease. The more electropositive surface potential of SUGARWIN1 would also be expected to favor complex formation with RNA. A phylogenetic analysis of PR-4 proteins obtained from 106 Embryophyta genomes showed that both catalytic histidines are widespread among them with few replacements in these amino acid positions during the gene family evolutionary history. We observe that the H11 replacement by N11 is also present in two other sugarcane PR-4 proteins: SUGARWIN3 and SUGARWIN4. We propose that RNase activity was present in the first Embryophyta PR-4 proteins but was recently lost in members of this family during the course of evolution @article={003041851,author = {MAIA, Lorhenn Bryanda Lemes; PEREIRA, Humberto d'Muniz; GARRATT, Richard Charles; BRANDÃO-NETO, José; SILVA, Flavio Henrique; TOYAMA, Danyelle; DIAS, Renata O; BACHEGA, José Fernando Ruggiero; PEIXOTO, Julia Vasconcellos; SILVA-FILHO, Marcio de Castro.},title={Structural and evolutionary analyses of PR-4 SUGARWINs points to a different pattern of protein function},journal={Frontiers in Plant Science},note={v. 12, art. 734248, p. 1-13},year={2021}}
@article={003041851,author = {MAIA, Lorhenn Bryanda Lemes; PEREIRA, Humberto d'Muniz; GARRATT, Richard Charles; BRANDÃO-NETO, José; SILVA, Flavio Henrique; TOYAMA, Danyelle; DIAS, Renata O; BACHEGA, José Fernando Ruggiero; PEIXOTO, Julia Vasconcellos; SILVA-FILHO, Marcio de Castro.},title={Structural and evolutionary analyses of PR-4 SUGARWINs points to a different pattern of protein function},journal={Frontiers in Plant Science},note={v. 12, art. 734248, p. 1-13},year={2021}}