Salmonella membrane structural remodeling increases resistance to antimicrobial peptide LL-37.
MARTYNOWYCZ, Michael W.; RICE, Amy; ANDREEV, Konstantin; NOBRE, Thatyane Morimoto; KUZMENKO, Ivan; WERESZCZYNSKI, Jeff; GIDALEVITZ, David.
MARTYNOWYCZ, Michael W.; RICE, Amy; ANDREEV, Konstantin; NOBRE, Thatyane Morimoto; KUZMENKO, Ivan; WERESZCZYNSKI, Jeff; GIDALEVITZ, David.
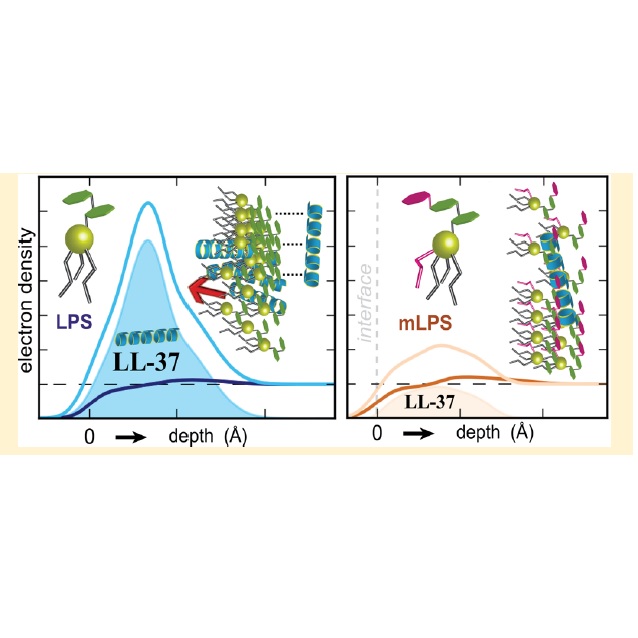 Abstract: Gram-negative bacteria are protected from their environment by an outer membrane that is primarily composed of lipopolysaccharides (LPSs). Under stress, pathogenic serotypes of Salmonella enterica remodel their LPSs through the PhoPQ two-component regulatory system that increases resistance to both conventional antibiotics and antimicrobial peptides (AMPs). Acquired resistance to AMPs is contrary to the established narrative that AMPs circumvent bacterial resistance by targeting the general chemical properties of membrane lipids. However, the specific mechanisms underlying AMP resistance remain elusive. Here we report a 2-fold increase in bacteriostatic concentrations of human AMP LL-37 for S. enterica with modified LPSs. LPSs with and without chemical modifications were isolated and investigated by Langmuir films coupled with grazing-incidence X-ray diffraction (GIXD) and specular X-ray reflectivity (XR). The initial interactions between LL-37 and LPS bilayers were probed using allatom molecular dynamics simulations. These simulations suggest that initial association is nonspecific to the type of LPS and governed by hydrogen bonding to the LPS outer carbohydrates. GIXD experiments indicate that the interactions of the peptide with monolayers reduce the number of crystalline domains but greatly increase the typical domain size in both LPS isoforms. Electron densities derived from XR experiments corroborate the bacteriostatic values found in vitro and indicate that peptide intercalation is reduced by LPS modification. We hypothesize that defects at the liquid-ordered boundary facilitate LL-37 intercalation into the outer membrane, whereas PhoPQ-mediated LPS modification protects against this process by having innately increased crystallinity. Since induced ordering has been observed with other AMPs and drugs, LPS modification may represent a general mechanism by which Gram-negative bacteria protect against host innate immunity. Abstract: Gram-negative bacteria are protected from their environment by an outer membrane that is primarily composed of lipopolysaccharides (LPSs). Under stress, pathogenic serotypes of Salmonella enterica remodel their LPSs through the PhoPQ two-component regulatory system that increases resistance to both conventional antibiotics and antimicrobial peptides (AMPs). Acquired resistance to AMPs is contrary to the established narrative that AMPs circumvent bacterial resistance by targeting the general chemical properties of membrane lipids. However, the specific mechanisms underlying AMP resistance remain elusive. Here we report a 2-fold increase in bacteriostatic concentrations of human AMP LL-37 for S. enterica with modified LPSs. LPSs with and without chemical modifications were isolated and investigated by Langmuir films coupled with grazing-incidence X-ray diffraction (GIXD) and specular X-ray reflectivity (XR). The initial interactions between LL-37 and LPS bilayers were probed using allatom molecular dynamics simulations. These simulations suggest that initial association is nonspecific to the type of LPS and governed by hydrogen bonding to the LPS outer carbohydrates. GIXD experiments indicate that the interactions of the peptide with monolayers reduce the number of crystalline domains but greatly increase the typical domain size in both LPS isoforms. Electron densities derived from XR experiments corroborate the bacteriostatic values found in vitro and indicate that peptide intercalation is reduced by LPS modification. We hypothesize that defects at the liquid-ordered boundary facilitate LL-37 intercalation into the outer membrane, whereas PhoPQ-mediated LPS modification protects against this process by having innately increased crystallinity. Since induced ordering has been observed with other AMPs and drugs, LPS modification may represent a general mechanism by which Gram-negative bacteria protect against host innate immunity. | |
| ACS Infectious Diseases |
| v. 5, n. 7, p. 1214-1222 - Ano: 2019 |
| Fator de Impacto: 4,911 |
| http://dx.doi.org/10.1021/acsinfecdis.9b00066 |  @article={002955253,author = {MARTYNOWYCZ, Michael W.; RICE, Amy; ANDREEV, Konstantin; NOBRE, Thatyane Morimoto; KUZMENKO, Ivan; WERESZCZYNSKI, Jeff; GIDALEVITZ, David.},title={Salmonella membrane structural remodeling increases resistance to antimicrobial peptide LL-37},journal={ACS Infectious Diseases},note={v. 5, n. 7, p. 1214-1222},year={2019}} @article={002955253,author = {MARTYNOWYCZ, Michael W.; RICE, Amy; ANDREEV, Konstantin; NOBRE, Thatyane Morimoto; KUZMENKO, Ivan; WERESZCZYNSKI, Jeff; GIDALEVITZ, David.},title={Salmonella membrane structural remodeling increases resistance to antimicrobial peptide LL-37},journal={ACS Infectious Diseases},note={v. 5, n. 7, p. 1214-1222},year={2019}} |





