Structure-function studies of BPP-BrachyNH2 and synthetic analogues thereof with angiotensin I-converting enzyme.
ARCANJO, Daniel D. R.; VASCONCELOS, Andreanne G.; NASCIMENTO, Lucas A.; MAFUD, Ana Carolina; PLÁCIDO, Alexandra; ALVES, Michel M. M.; DELERUE-MATOS, Cristina; BEMQUERER, Marcelo P.; VALE, Nuno; GOMES, Paula; OLIVEIRA, Eduardo Brandt de; LIMA, Francisco C. A.; MASCARENHAS, Yvonne Primerano; CARVALHO, Fernando Aécio A.; SIMONSEN, Ulf; RAMOS, Ricardo M.; LEITE, José Roberto S. A.
ARCANJO, Daniel D. R.; VASCONCELOS, Andreanne G.; NASCIMENTO, Lucas A.; MAFUD, Ana Carolina; PLÁCIDO, Alexandra; ALVES, Michel M. M.; DELERUE-MATOS, Cristina; BEMQUERER, Marcelo P.; VALE, Nuno; GOMES, Paula; OLIVEIRA, Eduardo Brandt de; LIMA, Francisco C. A.; MASCARENHAS, Yvonne Primerano; CARVALHO, Fernando Aécio A.; SIMONSEN, Ulf; RAMOS, Ricardo M.; LEITE, José Roberto S. A.
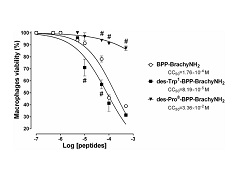 Abstract: The vasoactive proline-rich oligopeptide termed BPP-BrachyNH2 (H-WPPPKVSP-NH2) induces in vitro inhibitory activity of angiotensin I-converting enzyme (ACE) in rat blood serum. In the present study, the removal of N-terminal tryptophan or C-terminal proline from BPP-BrachyNH2 was investigated in order to predict which structural components are important or required for interaction with ACE. Furthermore, the toxicological profile was assessed by in silico prediction and in vitro MTT assay. Two BPP-BrachyNH2 analogues (des-Trp1-BPP-BrachyNH2 and des-Pro8-BPP-BrachyNH2) were synthesized, and in vitro and in silico ACE inhibitory activity and toxicological profile were assessed. The des-Trp1-BPP-BrachyNH2 and des-Pro8-BPP-BrachyNH2 were respectively 3.2- and 29.5-fold less active than the BPP-BrachyNH2-induced ACE inhibitory activity. Molecular Dynamic and Molecular Mechanics Poisson-Boltzmann Surface Area simulations (MM-PBSA) demonstrated that the ACE/BBP-BrachyNH2 complex showed lower binding and van der Wall energies than the ACE/des-Pro8-BPP-BrachyNH2 complex, therefore having better stability. The removal of the N-terminal tryptophan increased the in silico predicted toxicological effects and cytotoxicity when compared with BPP-BrachyNH2 or des-Pro8-BPP-BrachyNH2. Otherwise, des-Pro8-BPP-BrachyNH2 was 190-fold less cytotoxic than BPP-BrachyNH2. Thus, the removal of C-terminal proline residue was able to markedly decrease both the BPP-BrachyNH2-induced ACE inhibitory and cytotoxic effects assessed by in vitro and in silico approaches. In conclusion, the aminoacid sequence of BPP-BrachyNH2 is essential for its ACE inhibitory activity and associated with an acceptable toxicological profile. The perspective of the interactions of BPP-BrachyNH2 with ACE found in the present study can be used for development of drugs with differential therapeutic profile than current ACE inhibitors. Abstract: The vasoactive proline-rich oligopeptide termed BPP-BrachyNH2 (H-WPPPKVSP-NH2) induces in vitro inhibitory activity of angiotensin I-converting enzyme (ACE) in rat blood serum. In the present study, the removal of N-terminal tryptophan or C-terminal proline from BPP-BrachyNH2 was investigated in order to predict which structural components are important or required for interaction with ACE. Furthermore, the toxicological profile was assessed by in silico prediction and in vitro MTT assay. Two BPP-BrachyNH2 analogues (des-Trp1-BPP-BrachyNH2 and des-Pro8-BPP-BrachyNH2) were synthesized, and in vitro and in silico ACE inhibitory activity and toxicological profile were assessed. The des-Trp1-BPP-BrachyNH2 and des-Pro8-BPP-BrachyNH2 were respectively 3.2- and 29.5-fold less active than the BPP-BrachyNH2-induced ACE inhibitory activity. Molecular Dynamic and Molecular Mechanics Poisson-Boltzmann Surface Area simulations (MM-PBSA) demonstrated that the ACE/BBP-BrachyNH2 complex showed lower binding and van der Wall energies than the ACE/des-Pro8-BPP-BrachyNH2 complex, therefore having better stability. The removal of the N-terminal tryptophan increased the in silico predicted toxicological effects and cytotoxicity when compared with BPP-BrachyNH2 or des-Pro8-BPP-BrachyNH2. Otherwise, des-Pro8-BPP-BrachyNH2 was 190-fold less cytotoxic than BPP-BrachyNH2. Thus, the removal of C-terminal proline residue was able to markedly decrease both the BPP-BrachyNH2-induced ACE inhibitory and cytotoxic effects assessed by in vitro and in silico approaches. In conclusion, the aminoacid sequence of BPP-BrachyNH2 is essential for its ACE inhibitory activity and associated with an acceptable toxicological profile. The perspective of the interactions of BPP-BrachyNH2 with ACE found in the present study can be used for development of drugs with differential therapeutic profile than current ACE inhibitors. | |
| European Journal of Medicinal Chemistry |
| v. 139, p. 401-411 - Ano: 2017 |
| Fator de Impacto: 4,519 |
| http://dx.doi.org/10.1016/j.ejmech.2017.08.019 |  @article={002846419,author = {ARCANJO, Daniel D. R.; VASCONCELOS, Andreanne G.; NASCIMENTO, Lucas A.; MAFUD, Ana Carolina; PLÁCIDO, Alexandra; ALVES, Michel M. M.; DELERUE-MATOS, Cristina; BEMQUERER, Marcelo P.; VALE, Nuno; GOMES, Paula; OLIVEIRA, Eduardo Brandt de; LIMA, Francisco C. A.; MASCARENHAS, Yvonne Primerano; CARVALHO, Fernando Aécio A.; SIMONSEN, Ulf; RAMOS, Ricardo M.; LEITE, José Roberto S. A.},title={Structure-function studies of BPP-BrachyNH2 and synthetic analogues thereof with angiotensin I-converting enzyme},journal={European Journal of Medicinal Chemistry},note={v. 139, p. 401-411},year={2017}} @article={002846419,author = {ARCANJO, Daniel D. R.; VASCONCELOS, Andreanne G.; NASCIMENTO, Lucas A.; MAFUD, Ana Carolina; PLÁCIDO, Alexandra; ALVES, Michel M. M.; DELERUE-MATOS, Cristina; BEMQUERER, Marcelo P.; VALE, Nuno; GOMES, Paula; OLIVEIRA, Eduardo Brandt de; LIMA, Francisco C. A.; MASCARENHAS, Yvonne Primerano; CARVALHO, Fernando Aécio A.; SIMONSEN, Ulf; RAMOS, Ricardo M.; LEITE, José Roberto S. A.},title={Structure-function studies of BPP-BrachyNH2 and synthetic analogues thereof with angiotensin I-converting enzyme},journal={European Journal of Medicinal Chemistry},note={v. 139, p. 401-411},year={2017}} |





